目的 通过CT-MRI图像融合技术结合磁共振多模态成像对瘤区进行精确勾画,探讨该技术对颅脑恶性肿瘤在放疗中的作用及价值。方法 将14例颅脑恶性肿瘤患者的CT平扫图像及MR平扫和增强图像传送至后处理工作站,并结合MR多模态图像在CT-MRI融合图像上对肿瘤进行勾画,比较勾画区的肿瘤边界,分析瘤周水肿和神经纤维对肿瘤勾画的影响。结果 利用CT-MR图像融合技术并结合磁共振多模态成像勾画的计划放疗范围明显精确于单纯在CT图像上勾画的计划放疗范围,并能有效避开重要神经纤维、海马及脑干结构,减少了因单纯CT图
多模态磁共振成像及融合技术在颅脑恶性肿瘤放疗靶区勾画中的应用
王长福 王斌杰△ 王 智 周彦汝 贺 祥 石晓莹 姜澳田
河南大学淮河医院影像科 河南大学医学影像研究所,河南 开封 475000
基金项目:河南省医学科技攻关计划重点项目(201402025);河南省科技攻关计划(172102310157)
作者简介:王长福,Email:1974103323@qq.com
△通信作者:王斌杰,Email:wbinjie@126.com
【摘要】 目的 通过CT-MRI图像融合技术结合磁共振多模态成像对瘤区进行精确勾画,探讨该技术对颅脑恶性肿瘤在放疗中的作用及价值。方法 将14例颅脑恶性肿瘤患者的CT平扫图像及MR平扫和增强图像传送至后处理工作站,并结合MR多模态图像在CT-MRI融合图像上对肿瘤进行勾画,比较勾画区的肿瘤边界,分析瘤周水肿和神经纤维对肿瘤勾画的影响。结果 利用CT-MR图像融合技术并结合磁共振多模态成像勾画的计划放疗范围明显精确于单纯在CT图像上勾画的计划放疗范围,并能有效避开重要神经纤维、海马及脑干结构,减少了因单纯CT图像显示的肿瘤边界不清导致与放疗靶区较实际靶区的误差,从而使肿瘤放疗的局控率有了较大的提高。结论 利用CT-MR图像融合技术结合磁共振多模态成像可明显提高颅脑恶性肿瘤放疗靶区勾画的准确性及精确性,减少主观因素差异性。
【关键词】 脑肿瘤;恶性肿瘤;螺旋计算机;磁共振成像;放射治疗
【中图分类号】 R739.41 【文献标识码】 A 【文章编号】 1673-5110(2018)19-2118-07 DOI:10.12083/SYSJ.2018.19.462
The target profile of multimodal magnetic resonance imaging in the radiotherapy of craniocerebral malignant tumor
WANG Changfu,WANG Binjie,WANG Zhi,ZHOU Yanru,HE Xiang,SHI Xiaoying,JIANG Aotian
Department of Radiology,Huaihe Hospital,Henan University/The Research Institute of Medical Image in Henan University,Kaifeng 475000,China
【Abstract】 Objective To accurately delineate the tumor area by CT-MRI image fusion technique combined with magnetic resonance multimodal imaging,and to explore the role and value of this technique in the treatment of brain malignant tumors.Methods CT scan images and MR plain scan images and MR images of patients with malignant brain tumors were transmitted to the post-processing workstation.The tumors were delineated on CT-MRI fusion images in combination with MR multimodal images.Tumor borders were analyzed for the effect of peritumoral edema and nerve fibers on tumor delineation.Results The planned radiotherapy range using CT-MR image fusion technology combined with magnetic resonance multimodal imaging was significantly more accurate than the planned radiotherapy range outlined on CT images,and could effectively avoid important nerve fibers,hippocampus and brainstem structures.The tumor boundary which is displayed by the simple CT image is reduced,and the target area of the radiotherapy target area is larger than the actual target area,so that the local control rate of the tumor radiotherapy is greatly improved.Conclusion Using CT-MR image fusion technology combined with magnetic resonance multimodal imaging can significantly improve the accuracy and accuracy of delineation of radiotherapy targets in brain malignant tumors,and reduce the difference of subjective factors.
【Key words】 Brain neoplasms;Malignant neoplasms;Spiral computer;Magnetic resonance imaging;Radiotherapy
胶质瘤和转移瘤是颅内常见恶性肿瘤,手术切除和(或)术后放疗是首选的治疗方案。一直以来,计算机断层扫描(computed tomography,CT)图像仍是放疗中靶区和危及器官勾画的最佳图像资源,但由于CT的软组织分辨率较差,肿瘤边界模糊,即使采用CT增强仍会有部分病灶显示不清或不显示,对于瘤周水肿范围、水肿区有无肿瘤病灶及是否将神经纤维勾画进去等,CT图像更是无能为力。近年来,国内外利用CT-MRI图像融合技术对颅脑恶性肿瘤进行精确勾画的临床研究已逐步展开,并取得一定有信服力的成果,但方法较为单一。本研究通过CT-MR图像融合技术并结合磁共振多模态成像对瘤区进行精确勾画,以探讨该技术在颅脑恶性肿瘤在放疗中的作用及价值。
1 资料与方法
1.1 一般资料 本组病例是河南大学淮河医院2017-09—2018-08收治的脑胶质瘤患者14例,男6例,女8例;年龄50~73岁。患者为单发病变,均已行全切或不全切手术,KPS评分≥70分。WHO分级Ⅲ级3例,Ⅳ级11例,均有术后放疗指征。
1.2 设备和扫描参数 CT检查使用德国西门子公司产16排螺旋CT扫描仪,患者取仰卧位,机架零角度行颅脑横断位扫描,从延髓下缘扫描至颅顶,层厚3 mm,无间隔扫描。磁共振检查使用德国西门子公司产3.0T Verio超导型磁共振扫描仪,取仰卧位,用放疗定位架、头托及头模对头部进行固定,用腹部相控阵线圈覆盖头部。扫描范围自颅顶至延髓下缘,行颅脑平扫,横轴位用快速自旋回波序列(TSE) T2WI (TR 12 693 ms,TE 96 ms),层厚3 mm,层间距0 mm,矩阵320×320,视野(FOV)220 mm×220 mm,层数70层;快速小角度激发序列(flash 2d)T1WI压脂(TR 488 ms,TE 2.67 ms),层厚3 mm,层间距0 mm,矩阵320×320,视野(FOV)220 mm×220 mm,层数24层。同时辅以快速自旋回波序列(TSE) T2WI (TR 4 100 ms,TE 96 ms)矢状位成像。灌注及增强扫描,采用高压注射器经肘前静脉注入造影剂钆喷酸葡胺(Gd-DTPA),速率4.0 mL/s,剂量0.2 mL/kg。后行磁共振全脑灌注扫描及增强扫描,灌注扫描参数PWI (TR 1 800 ms,TE 30 ms),层厚3 mm,层间距0 mm,矩阵128×128 视野(FOV)230 mm×230 mm,层数24层;增强扫描参数,在T1WI脂肪抑制序列行横轴位扫描,T1WI (TR 1 400 ms,TE 2.67 ms),层厚3 mm,层间距0 mm,矩阵320×320 视野(FOV)230 mm×230 mm,层数70层。
1.3 MR多模态图像后处理 将磁共振波谱原始图像传输至磁共振工作站,经专用软件后处理后获得CBV(脑血容量)、CBF(脑血流量)、MTT(平均通过时间)、TTP(达峰时间)图像,观察病变及周围区域血流灌注情况。并将CBV与MR增强轴位图像融合,了解肿瘤术后边界及水肿范围。
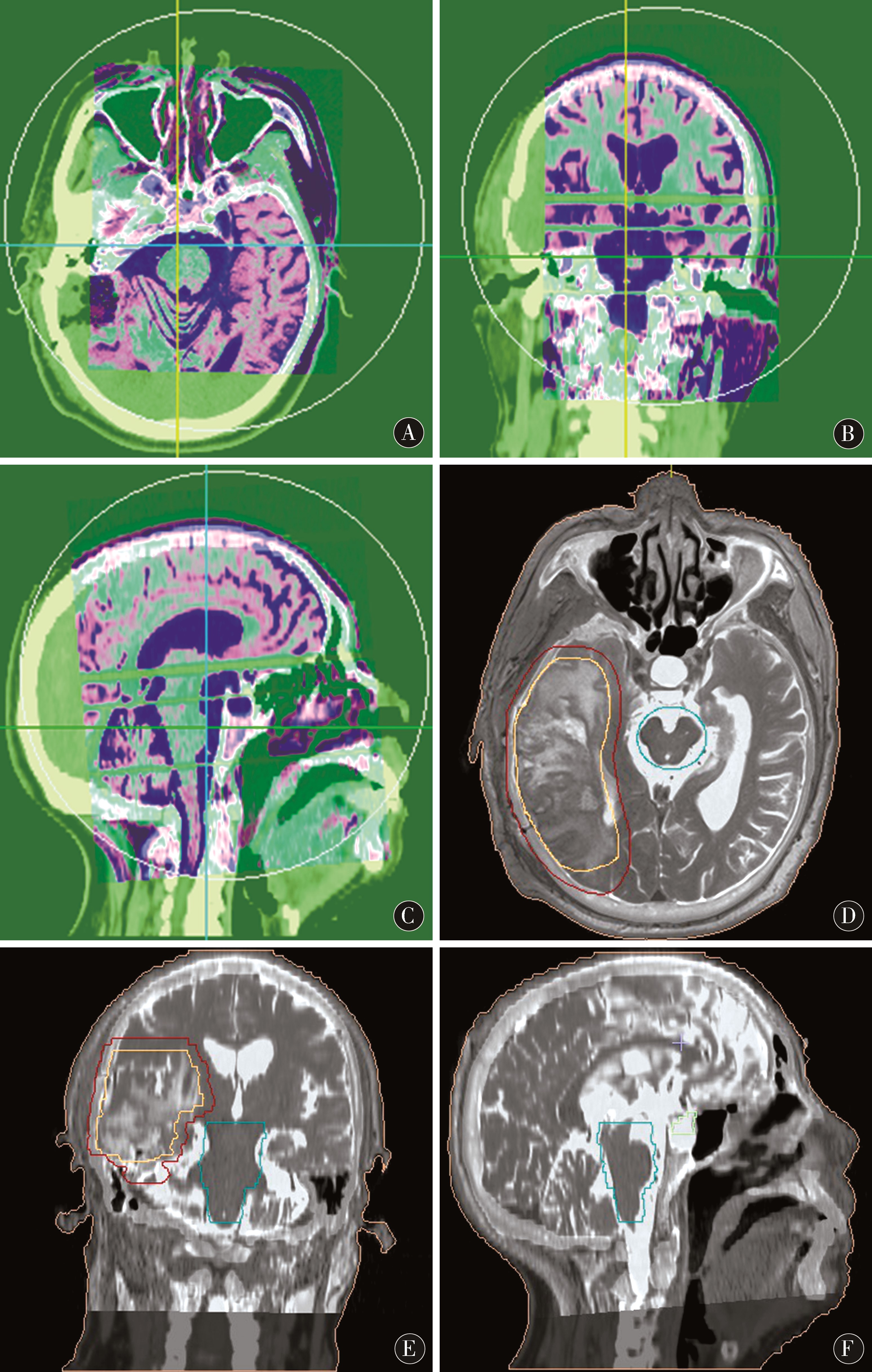
1.4 图像融合 分别将CT图像及磁共振平扫T2WI、增强轴位T1WI-FS、灌注CBV图像经院内局域网分别传送至放疗科FocalFusion工作站,将磁共振图像分别与CT定位图像进行图像配准融合(图1A~C),先进行匹配粗融合,再根据系统矫正功能对融合图像进行精细融合,使人体外轮廓、解剖结构及骨性标志等基本重合。融合效果由放疗科医师、物理师及影像科医师共同评估确定。最终生成CT/MRI-T2WI、CT/MRI-T1WI-FS及CT/MRI-CBV融合图像,用于靶区勾画。
图1 A~C:FocalFusion图像融合工作站进行CT/MRI图像融合配准;D~F:CT/MRI图像融合用于靶区勾画作治疗计划
Figure 1 A-C:FocalFusion image fusion workstation for CT/MRI image fusion registration;D-F:CT/MRI image fusion for target area delineation
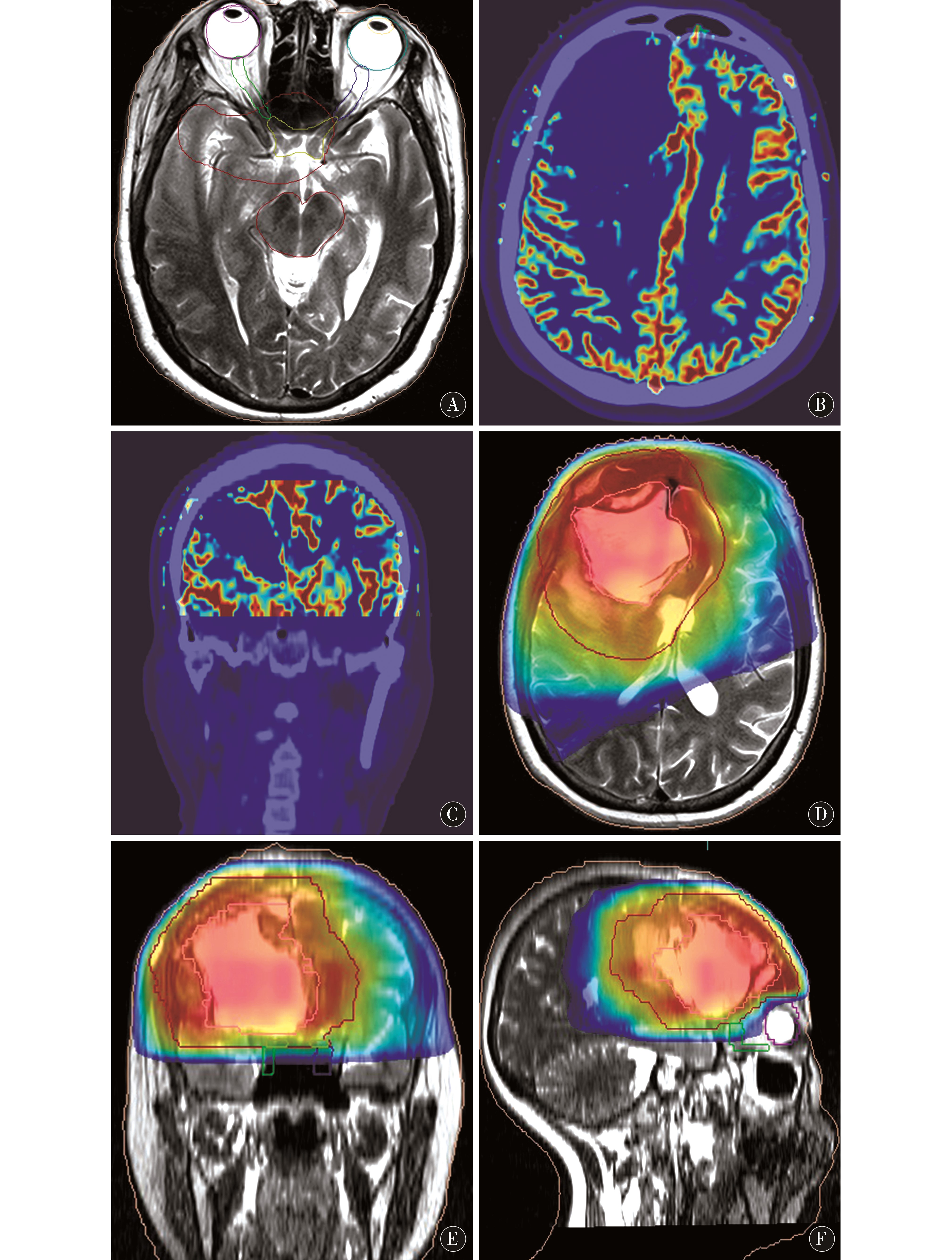
1.5 靶区勾画 先由放疗科医师在CT图像上对病变的放疗范围进行定位勾画。后由放疗科医师、物理师及影像科医师依据磁共振多模态图像提供的信息在CT/MRI-T2WI融合图像上勾画临床靶区(clinical target volume,CTV)(图1D~F),在CT/MRI-T1WI-FS、CT/MRI-CBV融合图像(图2B~C)、肿瘤浸润的天然屏障及重要部位(如视神经走形区、海马、脑干)处适当修改(图2A)。CT定位图上勾画的CTV命名为CTVCT,CT/MRI-T2WI图上勾画的CTV命名为CTVCT/MRI。将完成靶区勾画的CT文件传至计划系统作治疗计划,然后再将治疗计划回传至FocalFusion工作站结合磁共振图像对治疗计划进行二次评估(图2D~F)。
图2 A:在磁共振图像上对视神经、脑干重要结构进行剂量保护区域勾画;B~C:CT与磁共振灌注图像融合,用于判定有无肿瘤残留区域修订靶区;D~F:基于CT/MRI融合图像勾画靶区生成的放射治疗计划
Figure 2 A:Dose protection area for important structures of optic nerve and brainstem on magnetic resonance image Delineation;B-C:CT and magnetic resonance perfusion image fusion,used to determine the presence or absence of tumor residual area revised target area;D-F:based on CT /MRI fusion image delineation target area generated radiotherapy plan
1.6 观察指标 按图像分为CT定位图像组与CT/MRI图像融合组,利用治疗计划系统上靶区勾画体积计算功能分别计算CTV体积,观察不同组间靶区体积勾画的差异。
1.7 统计学方法 利用SPSS 23.0统计软件对数据进行统计分析,数据以均数±标准差(x±s)表示,组间比较采用配对t检验,以P<0.05为差异有统计学意义。
2 结果
放疗科医师勾画CTVCT和CTVCT/MRI体积[(342.54±63.22)cm3 vs(311.03±70.84)cm3]比较,差异有统计学意义(t=3.89,P=0.002)。
3 讨论
目前国际上放射治疗计划系统大部分是在立体定向、三维适形及静动态调强等方面进行的研究开发,并结合最新的计算机技术和快速精准的三维计量计算方法,以及多目标理论和算法开发精确放疗系统。这些计划系统所基于的2D/3D解剖结构图像,能够有效减少对解剖区域的放射损伤,最大限度减少辐射剂量,但不能保证对周围重要功能区域的保护进行可视化的精确指导。本课题旨在利用磁共振多模态技术对中枢神经系统肿瘤本身及周围神经结构及功能区进行可视化显示,并将其与CT图像融合,从而对病变区进行精确勾画,指导放射治疗计划的制定,达到精准放疗并有效保护正常组织的目的功能异常[1-2],同时也可以有效避免单纯MR图像的失真[3]。
在放射治疗实施过程中,靶区确定是首要且关键的步骤,医学影像技术是确定靶区的重要工具。CT成像一直以来都是放疗定位的重要工具,但由于其软组织分辨率较低,其对病灶的显示及对病变周围水肿范围的判断往往不准确。MR成像取决于物质的质子分布,对软组织特别是浸润性癌的显示更敏感,图像边界相对清晰,较CT影像能跟精确地描述肿块大小,对于肿瘤区级水肿区的判断也明显优于CT,并减少观察之间和观察者自身对肿瘤范围理解上的差异[4-10]。MRI对于脑转移瘤的诊断和GTV的确定明显优于CT,但由于MRI非线性的磁场和体内不同组织的磁化系数不同,存在图像的失真问题,更重要的是,MRI不能提供剂量所需的电子密度、阻止本领比等参数,加上扫描时间长和缺少固定装置,所以一直以来不用于治疗计划系统。而利用图像融合方式则充分发挥了MRI和CT的各自优势,对肿瘤的勾画更精细、更严谨[11-18]。
目前所指的多模态磁共振成像技术,包括磁共振扩散加权成像、扩散张量成像、灌注成像、波谱成像及血氧水平依赖的功能磁共振成像等,临床上可以根据需要对多模态成像技术进行自由组合及选择,以了解病变及周围组织的综合信息[19-30]。磁共振多模态成像与放射治疗计划系统相结合就是要充分利用计算机技术、人体组织解剖与功能的可视化、剂量计算手段、优化理论和算法、控制方法等,解决治疗计划的优化、剂量计算的快速精确、治疗过程的精确定位,并保证目标剂量的准确实施等问题,因此,磁共振多模态成像在放射治疗计划的制定方面有重要价值。
本研究采用脑灌注成像对颅脑肿瘤进行判定。该成像通过反映组织的微循环特点可以准确判断肿瘤性病变与非肿瘤性病变(如放射性脑病等),同时精确判断病变的大小、位置及边缘,了解其与神经纤维束的关系,以便精确勾画,准确定位,以达到对肿瘤进行精确放射治疗的同时能有效保护周围正常组织目的[31]。
本研究显示,利用CT-MRI融合技术并结合磁共振多模态成像勾画的计划放疗范围明显精确于单纯CT图像[32-35],且利用CT-MR多模态融合图像可以更有效地寻找肿瘤的边界、水肿范围、水肿区内有无肿瘤病灶及有效避开神经纤维束,最大程度避免放疗对海马的影响,其准确性和精确性均得到很大的提高,减少了因CT图像显示的肿瘤边界不清导致放疗靶区较实际靶区小的因素,从而是肿瘤放疗的局控率有了较大的提高[36-40]。
本研究创新点是在原有放疗计划基础上集成多模态神经成像,并用来修正放疗计划,在精准放射治疗,减少放射治疗并发症方面有重要意义。当前卫生部已把MR与CT图像融合用来指导放疗计划的制定列入一些中枢系统肿瘤的标准化治疗方案中,因此,磁共振多模态成像的对放射治疗计划的制定有重要意义。目前磁共振多模态成像技术,如MRI加权技术[41-43]、波谱技术[44-46]、弥散张量技术[47-51]及多模态3D打印技术[52]等已经广泛应用于脑肿瘤的精确诊断中,将这些技术与常规MR图像及与CT图像融合用于脑肿瘤放射治疗靶区勾画并护互为补充,将会使靶区勾画更精确、对人体造成的危害更小。
4 参考文献
[1] INOUE H K,NAKAJIMA A,SATO H,et al.Image Fusion for Radiosurgery,Neurosurgery and Hypofractionated Radiotherapy[J].Cureus,2015,7(3):e252-e270.
[2] ROBERT D,TIMMERMAN R D.An overview of hypofractionation and introduction to this issue of seminars in radiation oncology[J].Semin Radiat Oncol,2008,18(4):215-222.
[3] JONKER B P.Image fusion pitfalls for cranial radiosurgery[J].Surg Neurol Int,2013,4:123-128.
[4] LEIVA-SALINAS C,MUTTIKKAL T J E,FLORS L,et al.FDG PET/MRI Coregistration Helps Predict Response to Gamma Knife Radiosurgery in Patients With Brain Metastases[J].AJR Am J Roentgenol,2018 Nov 13:1-6.doi:10.2214/AJR.18.20006.
[5] LUKAS R V,JUHÁSZ C,WAINWRIGHT D A,et al.Imaging tryptophan uptake with positron emission tomography in glioblastoma patients treated with indoximod[J].J Neurooncol,2018 Nov 10.doi:10.1007/s11060-018-03013-x.
[6] QIU J,CUI Y,SUN L,et al.Hemichorea associated with cavernous angioma and a small errhysis:A case report and literature review[J].Medicine (Baltimore),2018,97(43):e12889.
[7] KESSLER A T,BHATT A A.Brain tumour post-treatment imaging and treatment-related complications[J].Insights Imaging,2018 Nov 8.doi:10.1007/s13244-018-0661-y.
[8] KRISTEVA M,SUPRUN A,GHAFFAR E,et al.Adult Medulloblastoma:Occurrence of a Rare Event[J].Cureus,2018,10(7):e3000.doi:10.7759/cureus.3000.
[9] HUANG R,CHEN Y,LI W,et al.An evidence-based approach to assess the accuracy of diffusion kurtosis imaging in characterization of gliomas[J].Medicine (Baltimore),2018,97(44):e13068.doi:10.1097/MD.0000000000013068.
[10] JIANG S,EBERHART C G,LIM M,et al.Identifying Recurrent Malignant Glioma after Treatment Using Amide Proton Transfer-Weighted MR Imaging:A Validation Study with Image-Guided Stereotactic Biopsy[J].Clin Cancer Res,2018 Oct 26.doi:10.1158/1078-0432.CCR-18-1233.
[11] SAJAMA C,LORENZONI J,TAGLE P.Diagnosis and treatment of brain metastasis[J].Rev Med Chil,2008,136(10):1 321-1 326.
[12] KONSKI A,DOSS M,MILESTONE B,et al.The integration of 18-fluoro-deoxy-glucose positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma[J].Int J Radiat Oncol Biol Phys,2005,61(4):1 123-1 128.
[13] 李洋,李香兰,王业伟,等.CT/MRI影像融合对肺癌脑转移放射治疗靶区勾画的影响[J].中国癌症杂志,2012,15(8):476-480.
[14] PANDITHARATNA E,KILBURN L B,ABOIAN M S,et al.Clinically Relevant and Minimally Invasive Tumor Surveillance of Pediatric Diffuse Midline Gliomas Using Patient-Derived Liquid Biopsy[J].Clin Cancer Res,2018 Oct 15.doi:10.1158/1078-0432.CCR-18-1345.
[15] NEUGUT A I,SACKSTEIN P,HILLYER G C,et al.Magnetic Resonance Imaging-Based Screening for Asymptomatic Brain Tumors:A Review[J].Oncologist,2018 Oct 10.doi:10.1634/theoncologist.2018-0177.
[16] MEOLA A,CHANG S D.Bilateral Vestibular Schwannomas in Neurofibromatosis Type 2[J].N Engl J Med,2018,379(15):1463.doi:10.1056/NEJMicm1804944.
[17] NEMATOLLAHI M,JAJROUDI M,ARBABI F,et al.The Benefits of Decision Tree to Predict Survival in Patients with Glioblastoma Multiforme with the Use of Clinical and Imaging Features[J].Asian J Neurosurg,2018,13(3):697-702.doi:10.4103/ajns.AJNS_336_16.
[18] SAWLANI V,DAVIES N,PATEL M,et al.Evaluation of Response to Stereotactic Radiosurgery in Brain Metastases Using Multiparametric Magnetic Resonance Imaging and a Review of the Literature[J].Clin Oncol (R Coll Radiol),2018 Sep 28.doi:10.1016/j.clon.2018.09.003.
[19] KEN S,VIEILLEVIGNE L,FRANCERIES X,et al.Integration method of 3D MR spectroscopy into treatment planning system for glioblastoma IMRT dose painting with integrated simultaneous boost[J].Radiat Oncol,2013,8:1.
[20] MONJAZEB A M,AYALA D,JENSEN C,et al.A phase Ⅰ dose escalation study of hypofractionated IMRT field-in-field boost for newly diagnosed glioblastoma multiforme[J].Int J Radiat Oncol Biol Phys,2012,82:743-748.
[21] BENTZEN S M,GREGOIRE V.Molecular imaging-based dose painting:a novel paradigm for radiation therapy prescription[J].Semin Radiat Oncol,2011,21:101-110.
[22] TSIEN C I,BROWN D,NORMOLLE D,et al.Concurrent temozolomide and dose-escalated intensity modulated radiation therapy in newly diagnosed glioblastoma[J].Clin Cancer Res,2012,18:273-279.
[23] SASAKI R,TANAKA N,OKAZAKI T,et al.Multiple cerebral syphilitic gummas mimicking brain tumor in a non-HIV-infected patient:A case report[J].J Infect Chemother,2018 Sep 21.doi:10.1016/j.jiac.2018.08.010.
[24] MORSHED R A,YOUNG J S,HAN S J,et al.Perioperative outcomes following reoperation for recurrent insular gliomas[J].J Neurosurg,2018 Sep 21:1-7.doi:10.3171/2018.4.JNS18375.
[25] BEIGI M,SAFARI M,AMERI A,et al.Findings of DTI-p maps in comparison with T(2)/T(2)-FLAIR to assess postoperative hyper-signal abnormal regions in patients with glioblastoma[J].Cancer Imaging,2018,18(1):33.doi:10.1186/s40644-018-0166-4.
[26] QI X L,YAO K,DUAN Z J,et al.BRAF V600E mutation and clinicopathologic characteristics in 250 cases of brain tumors associated with epilepsy[J].Zhonghua Bing Li Xue Za Zhi,2018,47(9):664-670.doi:10.3760/cma.j.issn.0529-5807.2018.09.003.
[27] NISHIKAWA M,INOUE A,OHNISHI T,et al.Significance of Glioma Stem-Like Cells in the Tumor Periphery That Express High Levels of CD44 in Tumor Invasion,Early Progression,and Poor Prognosis in Glioblastoma[J].Stem Cells Int,2018:5387041.doi:10.1155/2018/5387041.
[28] SEHGAL A A,LI Y,LAL B,et al.CEST MRI of 3-O-methyl-D-glucose uptake and accumulation in brain tumors[J].Magn Reson Me,2018 Sep 11.doi:10.1002/mrm.27489.
[29] XU X,ZHU H,LIU F,et al.Imaging Brain Metastasis Patients With 18F-(2S,4R)-4-Fluoroglutamine[J].Clin Nucl Med,2018,43(11):e392-e399.doi:10.1097/RLU.0000000000002257.
[30] YAMAMOTO J,KAKEDA S,SHIMAJIRI S,et al.Evaluation of Peritumoral Brain Parenchyma Using Contrast-Enhanced 3D Fast Imaging Employing Steady-State Acquisition at 3T for Differentiating Metastatic Brain Tumors and Glioblastomas[J].World Neuros-urg,2018 Aug 27.doi:10.1016/j.wneu.2018.08.147.
[31] KOGA T,SHIN M,MARUYAMA K,et al.Long-term outcomes of stereotactic radiosurgery for arteriovenous malformations in the thalamus[J].Neurosurg,2010,67:398-403.
[32] DJAN I,PETROVIÜ B,ERAK M,et al.Radiotherapy treatment planning:benefits of CT-MR image registration and fusion in tumor volume delineation[J].Vojnosanit Pregl,2013,70(8):735-739.
[33] KHOO V S,JOON D L.New developments in MRI for target vol-ume delineation in radiotherapy[J].Br J Radiol,2006,79 Spec No 1:S2-S15.
[34] SHARPE M,BROCK K K.Quality assurance of serial 3D image reg-istration,fusion,and segmentation[J].Int J Radiat Oncol Biol Phys,2008,71(1 Suppl):S33-S37.
[35] JANSEN E P,DEWIT L G,VAN HERK M,et al.Target volumes in radiotherapy for high-grade malignant glioma of the brain[J].Radiother Oncol,2000,56(2):151-156.
[36] FIORENTINO A,CAIVANO R,PEDICINI P,et al.Clinical target volume definition for glioblastoma radiotherapy planning:magnetic resonance imaging and computed tomography[J].Clin Transl Oncol,2013,15:754-758.
[37] BALDUCCI M,FIORENTINO A,DE BONIS P,et al.Impact of age and co-morbidities in patients with newly diagnosed glioblastoma:a pooled data analysis of three prospective mono-institutional phase Ⅱ studies[J].Med Oncol,2010,29(5):3 478-3 483.
[38] FARACE P,GIRI M G,MELIADO` G,et al.Clinical target volume delineation in glioblastomas:pre-operative versus post-operative/pre-radiotherapy MRI[J].Br J Radiol,2011,84:271-278.
[39] LAWRENCE Y R,LI XA,NAQA I,et al.Radiation dose-volume effects in the brain[J].Int J Radiat Oncol Biol Phys,76(3 Suppl):S20-S27.
[40] MINNITI G,AMELIO D,AMICHETTI M,et al.Patterns of failure and com-parison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolo-mide[J].Radiother Oncol,2010,97(3):377-381.
[41] 林坤,次单旺久,王晓明.多模态磁共振成像技术在胶质瘤细胞增值诊断中的应用[J].磁共振成像,2017,8(6):469-474.
[42] LOTUMOLO A,CAIVANO R,RABASCO P,et al.Comparison between magnetic resonance spectroscopy and diffusion weighted imaging in the evaluation of gliomas response after treatment[J].Eur J Radiol,2015,84(12):2 597-2 604.
[43] MABRAY M C,COHEN B A,VILLANUEVA-MEYER J E,et al.Performance of apparent diffusion coefficient values and conventional MRI features in differentiating tumefactive demyelinating lesions from primary brain neoplasms[J].AJR Am J Roentgenol,2015,205(5):1 075-1 085.
[44] WANG Q,ZHANG H,ZHANG J S,et al.The diagnostic performance of magnetic resonance spectroscopy in differentiating high-from lowgrade gliomas:a systematic review and meta-analysis[J].Eur Radiol,2016,26(8):2 670-2 684.
[45] FUDABA H,SHIMOMURA T,ABE T,et al.Comparison of multiple parameters obtained on 3.0 T pulsed arterial spin-labeling,diffusion tensor imaging,and MRS and the Ki-67 labeling index in evaluating glioma grading[J].AJNR Am J Neuroradiol,2014,35(11):2 091-2 098.
[46] SHANG H B,ZHAO W G,ZHANG W F.Preoperative assessment using multimodal functional magnetic resonance imaging techniques in patients with brain gliomas[J].Turk Neurosurg,2012,22(5):558-565.
[47] JIANG R F,JIANG J J,ZHAO L Y,et al.Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation[J].Oncotarget,2015,6(39):42 380-42 393.
[48] REN Y,PANG H P,FENG X Y,et al.Non-Gaussian diffusion MR imaging of glioma:comparisons of multiple diffusion parameters and correlation with histologic grade and MIB-1 (Ki-67 labeling) index[J].Neuroradiology,2016,58(2):121-132.
[49] TONOYAN A S,PRONIN I N,PITSKHELAURI D I,et al.A correlation between diffusion kurtosis imaging and the proliferative activity of brain glioma[J].Zh Vopr Neirokhir Im N N Burdenko,2015,79(6):5-14.
[50] SUI Y,XIONG Y,JIANG J,et al.Differentiation of Low-and High-Grade Gliomas Using High b-Value Diffusion Imaging with a Non-Gaussian Diffusion Model[J].AJNR Am J Neuroradiol,2016,37(9):1 643-1 649.
[51] 郭山山,王恩普,孙宏立.DTI在诊断中枢神经系统疾病的应用研究[J].中国实用神经疾病杂志,2017,20(4):55-57.
[52] 刘宇清,何炳蔚,庄江惠,等.基于颅脑CT与MRI多模图像融合的3D打印模型制作及应用研究[J].中国实用神经疾病杂志,2018,21(7):1 393-1 400.
(收稿2018-09-15 修回2018-09-30)
本文责编:夏保军
本文引用信息:王长福,王斌杰,王智,周彦汝,贺祥,石晓莹,姜澳田.多模态磁共振成像及融合技术在颅脑恶性肿瘤放疗靶区勾画中的应用[J].中国实用神经疾病杂志,2018,21(19):2118-2124.DOI:10.12083/SYSJ.2018.19.462
Reference information:WANG Changfu,WANG Binjie,WANG Zhi,ZHOU Yanru,HE Xiang,SHI Xiaoying,JIANG Aotian.The target profile of multimodal magnetic resonance imaging in the radiotherapy of craniocerebral malignant tumor[J].Chinese Journal of Practical Nervous Diseases,2018,21(19):2118-2124.DOI:10.12083/SYSJ.2018.19.462