目的 探讨术中磁共振(iMRI)联合神经导航在丘脑胶质瘤的应用价值。方法 回顾性分析2014-01—2017-02应用3.0T术中磁共振联合神经导航的28例丘脑胶质瘤手术患者的临床资料,评估术后肿瘤切除程度及术后功能状态。结果 28例患者第一次iMRI扫描17例仍有病变残留,进行扩大切除9例达到完整切除,全切率从39.3% 提高到71.4%,8例肿瘤边缘累及运动功能皮质或皮质脊髓束无法行完整切除。术后6个月28例丘脑胶质瘤患者神经功能改善者20例,无变化6例,下降2例。结论 术中磁共振联合神经导航有助于提高丘脑胶质瘤手术精准性和安全性,提高肿瘤切除程度,最小限度减少脑功能损伤,为术后手术效果提供帮助。
术中磁共振联合神经导航在丘脑胶质瘤手术中的应用
丁大领1) 赵爱玲2) 王朝艳3) 王树凯1) 刘献志1) 孙剑瑞1)△
1)郑州大学第一附属医院神经外科,河南郑州 450052 2)郑州大学附属儿童医院小婴儿科,河南郑州 450018 3)郑州大学第一附属医院磁共振科,河南郑州 450052
基金项目:河南省自然科学基金(编号:162300410311);郑州大学第一附属医院青年创新基金(编号:一附教科[2015]1号)
作者简介:丁大领,Email:dingdaling1982@163.com
△通信作者:孙剑瑞,Email:sunjianruizzu@163.com
【摘要】 目的 探讨术中磁共振(iMRI)联合神经导航在丘脑胶质瘤的应用价值。方法 回顾性分析2014-01—2017-02应用3.0T术中磁共振联合神经导航的28例丘脑胶质瘤手术患者的临床资料,评估术后肿瘤切除程度及术后功能状态。结果 28例患者第一次iMRI扫描17例仍有病变残留,进行扩大切除9例达到完整切除,全切率从39.3% 提高到71.4%,8例肿瘤边缘累及运动功能皮质或皮质脊髓束无法行完整切除。术后6个月28例丘脑胶质瘤患者神经功能改善者20例,无变化6例,下降2例。结论 术中磁共振联合神经导航有助于提高丘脑胶质瘤手术精准性和安全性,提高肿瘤切除程度,最小限度减少脑功能损伤,为术后手术效果提供帮助。
【关键词】 丘脑胶质瘤;颅内肿瘤;术中磁共振;神经导航;显微外科手术
【中图分类号】 R739.41 【文献标识码】 A 【文章编号】 1673-5110(2019)03-0253-07 DOI:10.12083/SYSJ.2019.03.049
Application of intraoperative magnetic resonance imaging combined with neuronavigation for operation of thalamic gliomas
DING Daling1),ZHAO Ailing2),WANG Chaoyan3),WANG Shukai1),LIU Xianzhi1),SUN Jianrui1)
1)Department of Neurosurgery,the First Affiliated Hospital of Zhengzhou University,Zhengzhou 450052,China;2) Department of Infant Ward,Children’s Hospital Affiliated of Zhengzhou University,Zhengzhou 450018,China;3) Department of MRI,the First Affiliated Hospital of Zhengzhou University,Zhengzhou 450052,China
【Abstract】 Objective To explore the clinical value ofintraoperative magnetic resonance imaging (iMRI) combined with neuronavigation on the operation of thalamic gliomas.Methods The clinical data of a total of 28 patients with thalamic glioma underwent microsurgical operation from January 2014 to February 2017 were retrospectively analyzed,and all patients aided by 3.0T iMRI combined with neuronavigation.The extent of resection of tumors and postoperative functional status were evaluated.Results In the first iMRI scan of 28 patients,residual tumors in 17 patients,9 patients achieved complete resection after received further extended resection,the total resection rate increased from 39.3% to 71.4%,and 8 tumors were unable to complete resection due to tumor edge involvement in motor function cortex or corticospinal tract.Six months after surgery,the neurological function with thalamus glioma improved or kept no change in 26 cases,and 2 cases experienced neurological function decline.Conclusions iMRI combined with neural navigation is helpful to improve the accuracy and safety of surgery for thalamus glioma,maximize the degree of tumor resection,minimize the damage to brain function,and provide help for postoperative operation recovery.
【Key words】 Thalamic gliomas;Intracranial tumor;Intraoperative magnetic resonance imaging;Neuronavigation;Microsurgery
丘脑肿瘤占颅内肿瘤的1%~5%,而胶质瘤占此部位的80%以上,从首发症状到确诊时间4~5个月,低级别胶质瘤以儿童及青少年为主,成人及中老年患者则多为高级别胶质瘤[1-4]。丘脑胶质瘤毗邻内囊、第三脑室及下丘脑等重要结构,其位置深,手术难度大,术后致死率及致残率高,所以对其治疗一直是神经外科的难题之一。手术切除是丘脑胶质瘤治疗的关键环节之一,其切除程度直接影响患者的预后和生活治疗[5-6]。近年来,由于神经导航技术以及术中磁共振(iMRI)的应用,丘脑胶质瘤的手术切除程度及术后疗效均明显提高,手术病残率与病死率大大降低[7-10]。本研究回顾性分析郑州大学第一附属医院神经外科应用术中磁共振联合神经导航指导手术切除的28例丘脑胶质瘤的临床资料。
1 资料和方法
1.1 一般资料 回顾性分析2014-01—2017-02郑州大学第一附属医院神经外科应用3.0T iMRI联合神经导航进行的28例丘脑胶质瘤手术患者的临床资料,男15例,女13例,年龄13~71(46.14±16.89)岁;儿童4例,成人24例;病史3 d~2 a,平均3.5个月。临床表现:大部分是亚急性或慢性病程,主要是偏瘫、感觉减退、癫痫、丘脑痛、不自主运动、步态紊乱及恶心呕吐等高颅压综合征。27例患者为首次手术,1例为复发胶质瘤二次手术。
1.2 方法 所有患者术前1 d在磁共振复合手术室行3.0T iMRI采集图像,包括平扫及增强序列,DTI序列及BOLD-fMRI等扫描。术前根据影像学表现、术者经验及神经导航计划,避开皮质功能区和纤维束等重要结构设计最佳的手术方案,如术腔肿瘤残留,更新导航参数,实时引导进一步切除,直至iMRI证实病变全切或达到术前计划的最大安全切除范围。
1.3 术后评估[11] (1)通过术前iMRI扫描精确定位丘脑肿瘤。(2)判断术后肿瘤切除程度。最后一次的iMRI扫描情况和术前丘脑肿瘤对比,根据RANO标准分为全切和部分切除。(3)观察患者术后新发运动情况,分为暂时性和永久性运动障碍。术前、术后1个月、术后6个月评估肌力及肌张力情况。
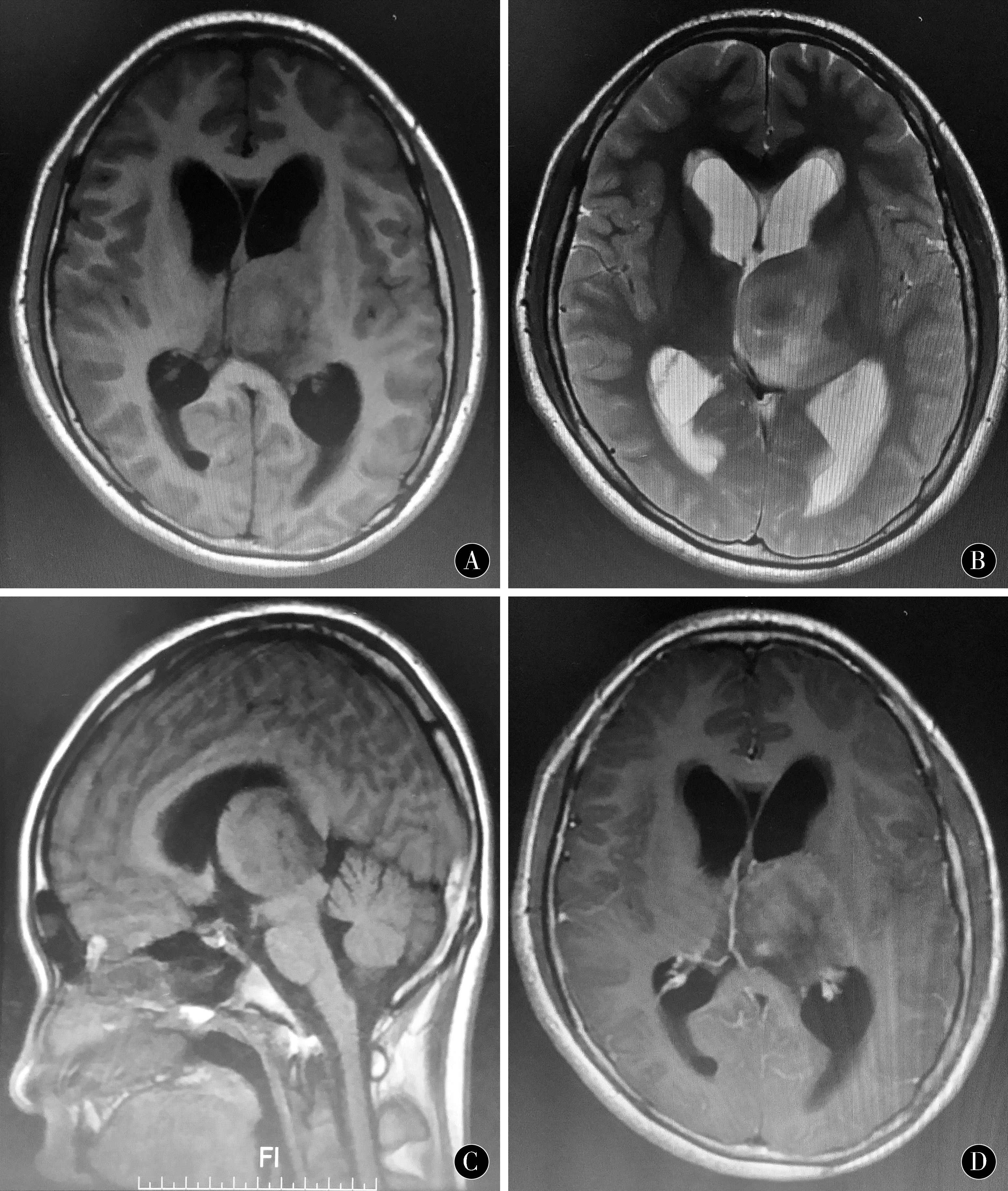
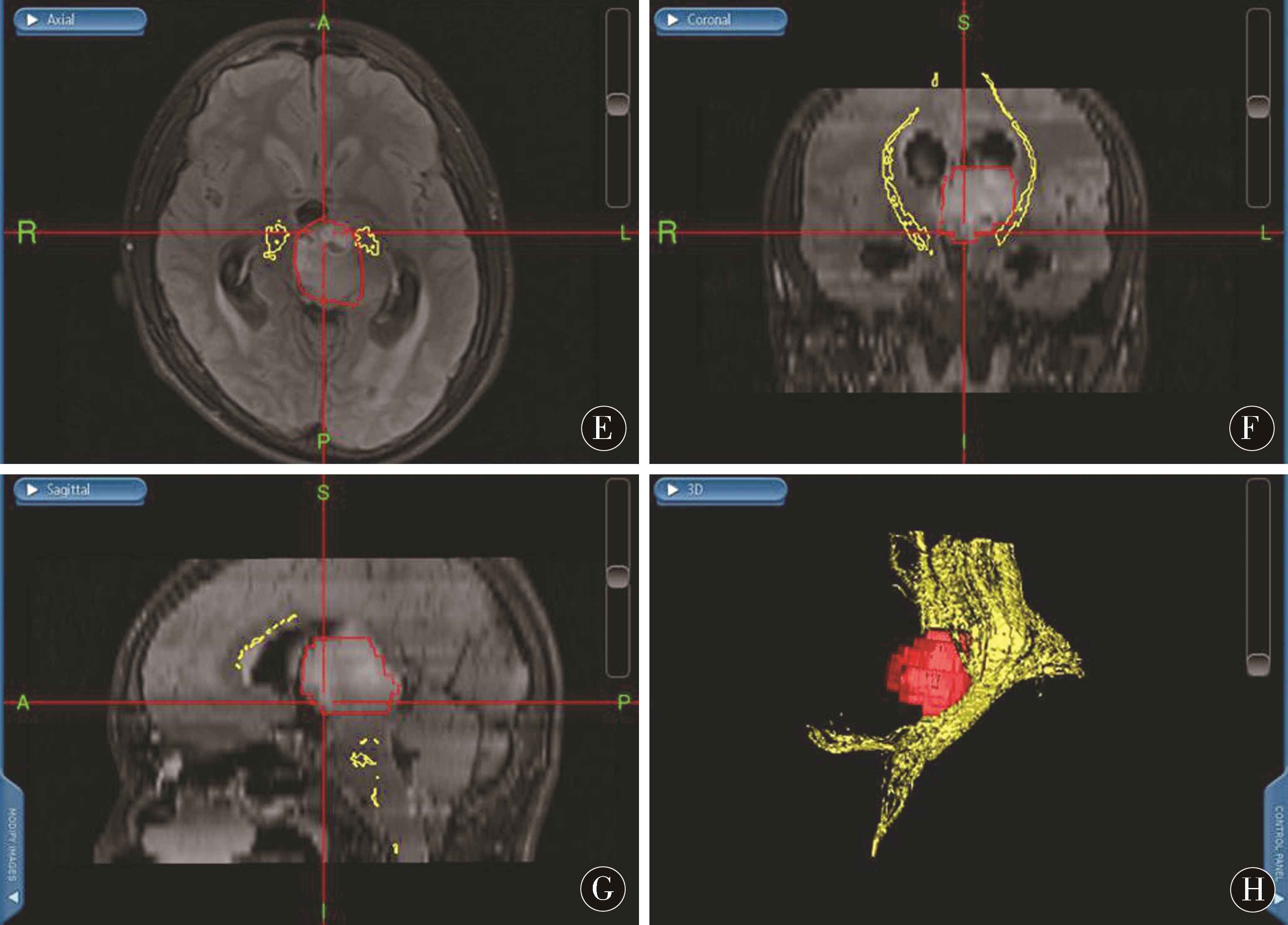
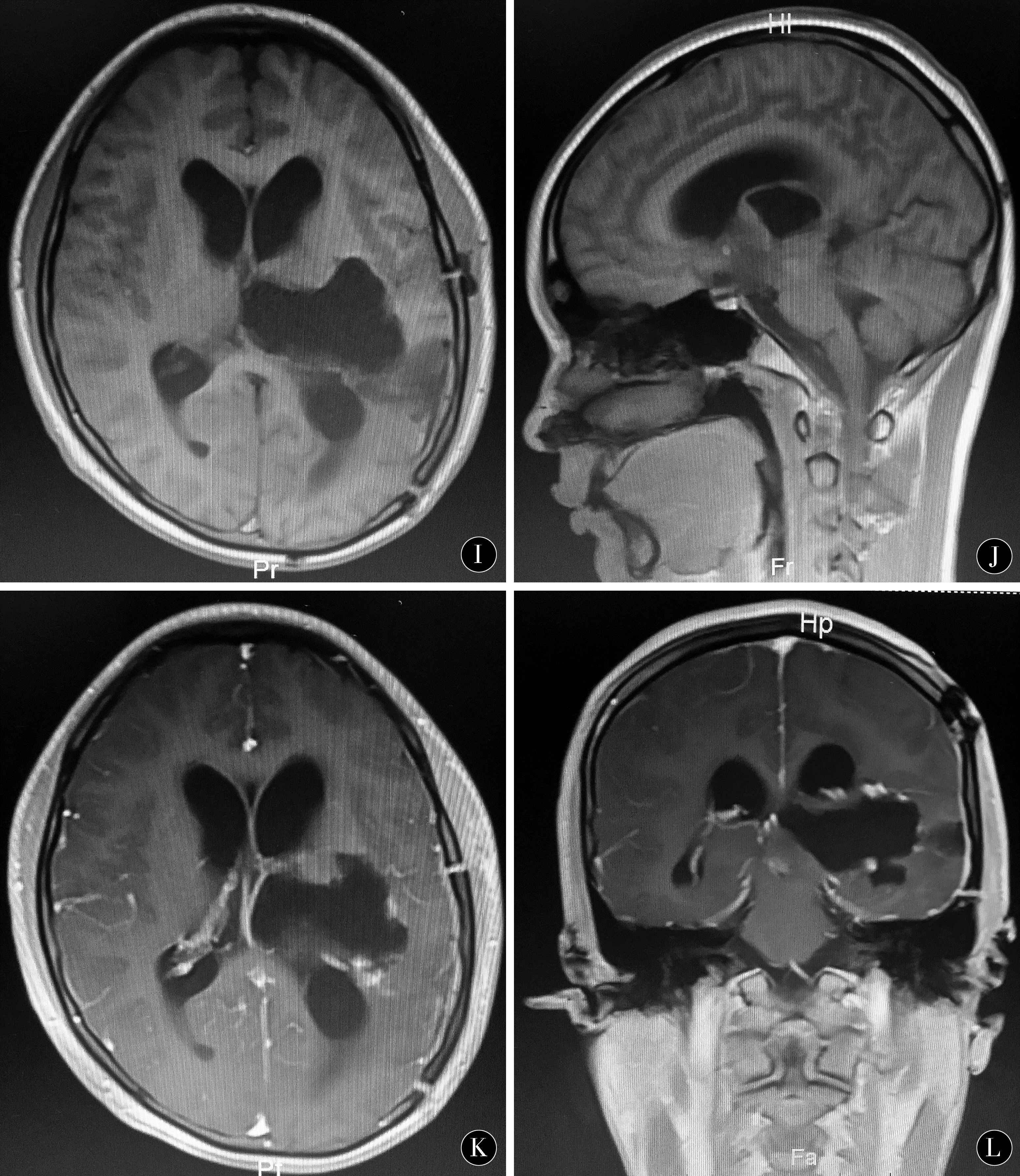
图1 左侧丘脑间变星形细胞瘤术前、术中及术后影像资料 A~D:术前MRI提示左侧丘脑病变;E~H:术中三维重建导航计划;I~L:术后MRI提示肿瘤全部切除
Figure 1 Left interthalamic astrocytoma preoperative,intraoperative and postoperative imaging data A-D:preoperative MRI suggest left thalamic lesions;E-H:intraoperative three-dimensional reconstruction navigation plan;I-L:surgery post-MRI suggests total tumor resection
2 结果
2.1 术中情况及病理结果 28例患者均在术中高场强磁共振联合神经功能导航下完成,手术过程顺利,无iMRI扫描及神经导航相关不良事件发生。本组病理学结果均为丘脑胶质瘤,根据WHO分级,WHO Ⅰ级2例,WHO Ⅱ级6例,WHO Ⅲ级11例,WHO Ⅳ级9例。
2.2 肿瘤切除情况 根据术前导航计划显示,20例丘脑肿瘤可完整切除,8例肿瘤边缘累及运动功能皮质或皮质脊髓束无法行完整切除。第一次iMRI扫描证实11例(39.3%)达到影像学全切除,17例仍有病变残留,9例继续进行扩大切除再经过iMRI扫描1~3次达到完整切除,全切率从39.3%提高到71.4%。考虑到术后功能的保留,8例未进一步切除,但切除程度达到预期目标。本研究中1例术中扫描术中发现原位血肿,急行血肿清除,术后患者意识清醒,无新发神经功能损害。
2.3 随访及预后结果 3个月~24个月,16例行术后60 Gy的放疗,18例采用替莫唑胺化疗。术后1个月与术前相比,神经功能改善者16例,无变化8例,下降4例(4例神经功能虽有所下降,但KPS评分>60,生活能够自理)。术后6个月神经功能改善者20例,无变化6例,下降2例。
3 讨论
丘脑胶质瘤是一种位于独特部位和复杂的临床过程的疾病,肿瘤周围为实质核团,虽然多出现膨胀性生长,但边界多清晰,好发于丘脑的后结节和前上部。特殊的解剖部位也导致丘脑胶质瘤的生长方式有以下三种情况:(1)胶质瘤局限于丘脑,周围的神经核团、内囊等重要组织结构受到侵袭破坏;(2)胶质瘤的生长超出丘脑范围,到达周围的脑叶及白质结构;(3)胶质瘤向侧脑室后角等部位生长,但未穿破脑室壁,所以根据胶质瘤生长部位、范围及神经纤维传导束和神经核团等破坏程度而表现不同的临床症状和体征[12]。但由于丘脑这一特殊部位和组织功能,导致丘脑胶质瘤预后相对较差,其规范化治疗方案也是神经外科医生面临的难题之一[13-15]。
随着科技的进步,神经外科的发展也越来越快,要求也越来越高,目前进入精准治疗时代,手术基本原则(3M原则)要求病变切除最大化、脑功能损伤最小化和术后恢复最佳化[16]。为追求这个目标,20世纪90年代中期,全球第一个术中MRI系统在美国哈佛大学医学院Brigham医院投入临床使用,Black等首次将术中磁共振应用于颅脑肿瘤的手术治疗中,取得较好的效果[17]。郑州大学第一附属医院神经外科自2012年引进iMRI应用于丘脑胶质瘤切除手术,也取得相对满意的效果。iMRI的优势:(1)提高手术切除率[18-23]:本组患者的全切率通过术中磁共振的应用明显提高,全切率从39.3% 提高到71.4%;(2)提高手术精准性和安全性[24-29]:通过iMRI实时扫描,不仅可以纠正术中脑脊液的流失和肿瘤切除造成神经导航的“漂移”,还可进行DTI等功能成像,从而提高手术安全性;(3)可及时发现残留肿瘤甚至新发血肿,避免二次手术造成的神经功能损害。本研究中1例术中扫描术中发现原位血肿,急行血肿清除,术后患者意识清醒,无新发神经功能损害;(4)iMRI实时导航选择最佳的手术入路:术前MRI扫描联合神经导航可重建肿瘤、锥体束等重要结构,从而选择合理的手术入路,术中操作做到心中有数[30-34]。本组病例通过iMRI实时导航,神经功能改善者16例。
对于丘脑胶质瘤患者除术前iMRI的相关准备外,术中也有诸多要点和细节问题需要注意。首先,选择合适的入路,剪开硬脑膜,释放脑脊液后,术者显微镜下要结合iMRI图像正确判断肿瘤、浸润脑组织和周围水肿带。术中减少对周围脑组织的过度牵拉,减少对神经纤维传导束和神经核团等重要结构的损伤,与非功能区胶质瘤切除不同。其次,进入侧脑室体部后,以丘纹静脉作为丘脑背外侧界的解剖标志,其外侧是豆状核和内囊部位,术中要注意功能保护。显微镜下可用棉片将侧脑室与肿瘤隔开,以防术中血液进入脑脊液循环,降低脑积水的发生。术中可先行瘤内切除,待肿瘤体积减小后再分离肿瘤的边缘[35-36]。再次,术中降低双极电凝功率,减少对周围重要结构的灼烧,对肿瘤内反复出血时可稍扩大切除至肿瘤边缘,并用明胶海绵和棉片压迫止血。最后,对于术后出现脑积水的患者,可尽早行脑室造瘘或分流手术。
术中磁共振联合神经导航有助于优选丘脑胶质瘤的手术入路,提高手术精准性和安全性[37-47],能最大程度切除丘脑胶质瘤,并最小限度减少脑功能的损伤,为提高手术效果和改善患者预后提供有力的支持和帮助。
4 参考文献
[1] 郑建,刘凤强.立体定向125I放射性粒籽植入术治疗成人丘脑高级别胶质瘤[J].中华医学杂志,2018,98(29):2 327-2 330.
[2] 李庆志,王鹏程,赵建农.两种显微手术入路切除丘脑胶质瘤[J].海南医学,2017,28(17):2 870-2 871.
[3] BUSH N A,CHANG S M,BERGER M S.Current and future strategies for treatment of glioma[J].Neurosurg Rev,2017,40(1):1-14.
[4] KRISHNATRY R,ZHUKOVA N,GUERREIRO SRUCKLIN A S,et al.Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma:a population-based study[J].Cancer,2016,122(8):1 261-1 269.
[5] 陈伟,杜昌旺,王茂德,等.显微手术治疗丘脑胶质瘤患者的生存状况研究[J].医学临床研究,2016,33(9):1 705-1 707.
[6] MCGIRT M J,MUKHERJEE D,CHAICHANA K L,et al.Association of surgically acquired motor and language deficits on overall survival after resection of glioblas-toma multiforme[J].Neurosurgery,2009,65(3):463-469.
[7] HOLLON T,STUMMER W,ORRINGER D,et al.Surgical adjuncts to increase the extent of resection:intraoperative MRI,fluorescence,and raman histology[J].Neurosurg Clin N Am,2019,30(1):65-74.
[8] AKBARI S H A,SYLVESTER P T,KULWIN C,et al.Initial experience using intraoperative magnetic resonance imaging during a trans-sulcal tubular retractor approach for the resection of deep-seated brain tumors:a case series[J].Oper Neurosurg (Hagerstown),2019,16(3):292-301.
[9] LEROY H A,DELMAIRE C,LE RHUN E,et al.High-field intraoperative MRI in glioma surgery:A prospective study with volumetric analysis of extent of resection and functional outcome[J].Neurochirurgie,2018,64(3):155-160.
[10] RAO G.Intraoperative MRI and maximizing extent of resection[J].Neurosurg Clin N Am,2017,28(4):477-485.
[11] 阎静,程敬亮,李红伟,等.术中磁共振联合运动功能神经导航在颅内病变切除术中的临床应用[J].郑州大学学报(医学版),2016,51(2):144-149.
[12] 伍碧武,张义,汪洋,等.显微外科手术治疗丘脑胶质瘤及预后分析[J].中华神经外科杂志,2015,31(12):1 201-1 205.
[13] SAI KIRAN N A,THAKAR S,DADLANI R,et al.Surgical management of thalamic gliomas:case selection,technical considerations,and review of literature[J].Neurosurg Rev,2013,36(3):383-393.
[14] WU B,TANG C,WANG Y,et al.High-grade thalamic gliomas:microsurgical treatment and prognosis analysis[J].J Clin Neurosci,2018,49:56-61.
[15] GUPTA A,SHALLER N,MCFADDEN K A.Pediatric thalamic gliomas:an updated review[J].Arch Pathol Lab Med,2017,141(10):1 316-1 323.
[16] 郭付有,王朝艳,宋来君,等.术中3.0T磁共振指导显微切除脑重要功能区病灶的应用[J].中华脑科疾病与康复杂志(电子版),2014,4(5):60-61.
[17] BLACK P M,ALEXANDER E,MARTIN C,et al.Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit[J].Neurosurgery,1999,45(3):431-433.
[18] RODER C,BISDAS S,EBNER F H,et al.Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery:high-field iMRI versus conventional and 5-ALA-assisted surgery[J].Eur J Surg Oncol,2014,40(3):297-304.
[19] STUPP R,TAILLIBERT S,KANNER A A,et al.Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma:a randomized clinical trial[J].JAMA,2015,314(23):2 535-2 543.
[20] ZHENG X,XU X,ZHANG H,et al.A preliminary experience with use of intraoperative magnetic resonance imaging in thalamic glioma surgery:a case series of 38 patients[J].World Neurosurg,2016,89:434-441.
[21] WHITING B B,LEE B S,MAHADEV V,et al.Combined use of minimal access craniotomy,intraoperative magnetic resonance imaging,and awake functional mapping for the resection of gliomas in 61 patients[J].J Neurosurg,2019,25:1-9.
[22] LU C Y,CHEN X L,CHEN X L,et al.Clinical application of 3.T intraoperative magnetic resonance combined with multimodal neuronavigation in resection of cerebral eloquent area glioma[J].Medicine(Baltimore),2018,97(34):e11702.
[23] EBELING M,LDEMANN W,FRISIUS J,et al.Venous thromboembolic complications with and without intermittent intraoperative and postoperative pneumatic compression in patients with glioblastoma multiforme using intraoperativemagnetic resonance imaging.a retrospective study[J].Neurochirurgie,2018,64(3):161-165.
[24] TRAYNOR C,HECKEMANN R A,HAMMER A,et al.Reproducibility of thalamic segmentation based on probabilistic tractography[J].Neuroimage,2010,52(1):69-85.
[25] SHAHAR T,ROZOVSKI U,MARKO N F,et al.Preoperative imaging to predict intraoperative changes in tumor-to-corticospinal tract distance:an analysis of 45 cases using high-field intraoperative magnetic resonance imaging[J].Neurosurgery,2014,75(1):23-30.
[26] POTGIESER A R,WAGEMAKERS M,VAN HULZEN A L,et al.The role of diffusion tensor imaging in brain tumor surgery:a review of the literature[J].Clin Neurol Neurosurg,2014,124:51-58.
[27] SENFT C,FORSTER M T,BINK A,et al.Optimizing the extent of resection in eloquently located gliomas by combining intraoperative MRI guidance with intraoperative neurophysiological monitoring[J].J Neurooncol,2012,109(1):81-90.
[28] PAMIR M N,ZDUMAN K,YILDIZ E,et al.Intraoperative magnetic resonance spectroscopy for identification of residual tumor during low-grade glioma surgery:clinical article[J].J Neurosurg,2013,118(6):1 191-1 198.
[29] MOHAMMADI A M,SULLIVAN T B,BARNETT G H,et al.Use of high field intraoperative magnetic resonance imaging to enhance the extent of resection of enhancing and nonenhancing gliomas[J].Neurosurgery,2014,74(4):339-348.
[30] 吴东东,陈晓雷,耿杰峰,等.术中高场强磁共振联合锥体束导航在丘脑胶质瘤切除手术中的应用[J].解放军医学院学报,2015,36(7):694-698.
[31] MOSHEL Y A,ELLIOTT R E,MONOKY D J,et al.Role of diffusion tensor imaging in resection of thalamic juvenile pilocytic astrocytoma[J].J Neurosurg Pediatr,2009,4(6):495-505.
[32] TAURA M,HAYASHI M,KONISHI Y,et al.Advanced image coregistration within the leksell workstation for the planning of glioma surgery:initial experience[J].J Neurol Surg Rep,2013,74(2):118-122.
[33] KIS D,MT A,KINCSES Z T,et al.The role of probabilistic tractography in the surgical treatment of thalamic gliomas[J].Neurosurgery,2014,10(Suppl 2):262-272.
[34] OZAWA N,MURAGAKI Y,NAKAMURA R,et al.Shift of the pyramidal tract during resection of the intraaxial brain tumors estimated by intraoperative Diffusion-Weighted imaging[J].Neurol Med Chir(Tokyo),2009,49(2):51-56.
[35] 张义,庄冬晓,张海石,等.经额叶侧脑室脉络膜裂入路丘脑胶质瘤切除术[J].中华神经外科杂志,2011,27(4):384-386.
[36] 李鑫,张鹏飞,韩利江,等.成人丘脑恶性胶质瘤手术治疗[J].中华神经外科疾病研究杂志,2017,16(2):146-150.
[37] MAJCHRZAK K,BOBEK-BILLEWICZ B,HEBDA A,et al.Surgical treatment of adult patients with thalamic tumors with the aid of tractography,fMRI,transcranial electrical stimulation and direct electrical stimulation of the subcortical white matter[J].Neurol Neurochir Pol,2018,52(6):720-730.doi:10.1016/j.pjnns.2018.07.001.
[38] CINALLI G,AGUIRRE D T,MIRONE G,et al.Surgical treatment of thalamic tumors in children[J].J Neurosurg Pediatr,2018,21(3):247-257.doi:10.3171/2017.7.PEDS16463.
[39] BROKINKEL B,YAVUZ M,WARNEKE N,et al.Endoscopic management of a low-grade thalamic glioma:a safe alternative to open microsurgery?[J].Acta Neurochir (Wien),2017,159(7):1 237-1 240.doi:10.1007/s00701-017-3120-5.
[40] NISHIHARA M,TAKEDA N,HARADA T,et al.Diagnostic yield and morbidity by neuronavigation-guided frameless stereotactic biopsy using magnetic resonance imaging and by frame-based computed tomography-guided stereotactic biopsy[J].Surg Neurol Int,2014,5(Suppl 8):S421-S426.doi:10.4103/2152-7806.140211.
[41] KIS D,MT A,KINCSES Z T,et al.The role of probabilistic tractography in the surgical treatment of thalamic gliomas[J].Neurosurgery,2014,10 Suppl 2:262-272;discussion 272.doi:10.1227/NEU.0000000000000333.
[42] MESSING-JNGER A M,FLOETH F W,PAULEIT D,et al.Multimodal target point assessment for stereotactic biopsy in children with diffuse bithalamic astrocytomas[J].Childs Nerv Syst,2002,18(8):445-449.
[43] CORRIVETTI F,DE SCHOTTEN M T,POISSON I,et al.Dissociating motor-speech from lexico-semantic systems in the left frontal lobe:insight from a series of 17 awake intraoperative mappings in glioma patients[J].Brain Struct Funct,2019 Jan 14.doi:10.1007/s00429-019-01827-7.
[44] TOVAR-SPINOZA Z,CHOI H.MRI-guided laser interstitial thermal therapy for the treatment of low-grade gliomas in children:a case-series review,description of the current technologies and perspectives[J].Childs Nerv Syst,2016,32(10):1 947-1 956.doi:10.1007/s00381-016-3193-0.
[45] CARRABBA G,BERTANI G,COGIAMANIAN F,et al.Role of Intraoperative Neurophysiologic Monitoring in the Resection of Thalamic Astrocytomas[J].World Neurosurg,2016,94:50-56.doi:10.1016/j.wneu.2016.06.049.
[46] ZHENG X,XU X,ZHANG H,et al.A Preliminary Experience with Use of Intraoperative Magnetic Resonance Imaging in Thalamic Glioma Surgery:A Case Series of 38 Patients[J].World Neurosurg,2016,89:434-441.doi:10.1016/j.wneu.2016.01.092.
[47] BROADWAY S J,OGG R J,SCOGGINS M A,et al.Surgical management of tumors producing the thalamopeduncular syndrome of childhood[J].J Neurosurg Pediatr,2011,7(6):589-595.doi:10.3171/2011.4.PEDS119.
(收稿2019-01-15)
本文责编:夏保军
本文引用信息:丁大领,赵爱玲,王朝艳,王树凯,刘献志,孙剑瑞.术中磁共振联合神经导航在丘脑胶质瘤手术中的应用[J].中国实用神经疾病杂志,2019,22(3):253-259.DOI:10.12083/SYSJ.2019.03.049
Reference information:DING Daling,ZHAO Ailing,WANG Chaoyan,WANG Shukai,LIU Xianzhi,SUN Jianrui.Application of intraoperative magnetic resonance imaging combined with neuronavigation for operation of thalamic gliomas[J].Chinese Journal of Practical Nervous Diseases,2019,22(3):253-259.DOI:10.12083/SYSJ.2019.03.049