目的 研究脑卒中患者血清人帕金森蛋白7(PARK7)、非对称二甲基精氨酸(ADMA)水平与神经功能缺损程度的关系。方法 2017-01-01—2018-01-01至广西中医药大学附属瑞康医院就诊的脑卒中随机选取患者82例,同时随机选择非脑卒中患者82例为对照组。对卒中患者组进行量化评分分为轻、中及重度损害组各48例、20例、14例。所有卒中组患者入院后均给予脱水、降颅压、保持呼吸道通畅及预防并发症等对症处理,分别测定2组血清PARK7、ADMA水平作以参考。结果 对比2组血清PARK7水平,轻度、中度及重度损害组分别为(20.19±4.21)μg/L、(27.68±4.53)μg/L及(33.74±5.27)μg/L,均明显高于对照组,差异有统计学意义(P<0.05),且3组间对比差异有统计学意义(P<0.05);对比2组血清ADMA水平,发现轻、中及重度损害组分别为(50.24±10.28)μmol/L、(74.72±11.84)μmol/L及(91.71±10.49)μmol/L,均明显高于对照组,2组比较差异有统计学意义(P<0.05)。Speaman等级相关和Pearson相关性分析显示,2组血清学数据与脑卒中患者神经功能相关评分严重程度呈正相关变化。即神经功能缺损越严重,其血清PARK7、ADMA水平亦越高(rPARK7=0.996 7,PPARK7=0.003 3;rADMA=0.999 7,PADMA=0.000 3),差异有统计学意义(P<0.05)。结论 脑卒中患者通常表现为不同程度的神经功能缺损,其血清PARK7、ADMA水平亦呈正相关变化,或可为脑卒中的早期诊疗提供理论依据,对不同程度脑卒中转归的判断具有一定指导作用,有利于治疗及病情评估。
脑卒中患者血清PARK7及ADMA水平与神经功能缺损的关系
曹 康1) 张永全2)△ 蔡碧清1) 胡洒洒1) 潘珠娣1)
1)广西中医药大学,广西南宁 530000 2)广西中医药大学附属瑞康医院神经内科二区,广西南宁 530000
基金项目:广西卫生厅中医药管理局自筹课题(编号:GZZC14-31);广西教育厅自筹课题(编号:KY2015LX160)
作者简介:曹康,Email:gemini_ck@qq.com
△通信作者:张永全,Email:gxzyq@126.com
【摘要】 目的 研究脑卒中患者血清人帕金森蛋白7(PARK7)、非对称二甲基精氨酸(ADMA)水平与神经功能缺损程度的关系。方法 2017-01-01—2018-01-01至广西中医药大学附属瑞康医院就诊的脑卒中随机选取患者82例,同时随机选择非脑卒中患者82例为对照组。对卒中患者组进行量化评分分为轻、中及重度损害组各48例、20例、14例。所有卒中组患者入院后均给予脱水、降颅压、保持呼吸道通畅及预防并发症等对症处理,分别测定2组血清PARK7、ADMA水平作以参考。结果 对比2组血清PARK7水平,轻度、中度及重度损害组分别为(20.19±4.21)μg/L、(27.68±4.53)μg/L及(33.74±5.27)μg/L,均明显高于对照组,差异有统计学意义(P<0.05),且3组间对比差异有统计学意义(P<0.05);对比2组血清ADMA水平,发现轻、中及重度损害组分别为(50.24±10.28)μmol/L、(74.72±11.84)μmol/L及(91.71±10.49)μmol/L,均明显高于对照组,2组比较差异有统计学意义(P<0.05)。Speaman等级相关和Pearson相关性分析显示,2组血清学数据与脑卒中患者神经功能相关评分严重程度呈正相关变化。即神经功能缺损越严重,其血清PARK7、ADMA水平亦越高(rPARK7=0.996 7,PPARK7=0.003 3;rADMA=0.999 7,PADMA=0.000 3),差异有统计学意义(P<0.05)。结论 脑卒中患者通常表现为不同程度的神经功能缺损,其血清PARK7、ADMA水平亦呈正相关变化,或可为脑卒中的早期诊疗提供理论依据,对不同程度脑卒中转归的判断具有一定指导作用,有利于治疗及病情评估。
【关键词】 脑卒中;人帕金森蛋白7;DJ-1蛋白质;非对称二甲基精氨酸;血清;神经功能缺损
【中图分类号】 R743.3 【文献标识码】 A 【文章编号】 1673-5110(2019)03-0260-07 DOI:10.12083/SYSJ.2019.03.050
Relationship between serum PARK7,ADMA and neurological deficit in stroke patients
CAO Kang1),ZHANG Yongquan2),CAI Biqing1),HU Sasa1),PAN Zhudi1)
1) Guangxi University of Traditional Chinese Medicine,Nanning 530000,China;2) Department of Neurology,Ruikang Hospital,Guangxi University of Traditional Chinese Medicine,Nanning 530000,China
【Abstract】 Objective To study the relationship between serum human Parkinson's protein 7 (PARK7),asymmetric dimethylarginine (ADMA) levels and neurological deficits in stroke patients.Methods A total of 82 stroke patients were randomly selected in the Ruikang Hospital Affiliated to Guangxi University of Traditional Chinese Medicine.82 patients with non-stroke were randomly selected as the control group.The quantitative scores of the stroke patients were divided into 48 cases of mild injury group,20 cases of moderate damage group and 14 cases of severe damage group.All stroke patients were treated with dehydration,intracranial pressure reduction,airway patency and prevention of complications after admission.The levels of PARK7 and ADMA in serum were determined for reference.Results Comparing the levels of PARK7 in serum,the patients with mild,moderate and severe damage were found to be (20.19±4.21)μg/L,(27.68±4.53)μg/L and (33.74±5.27)μg/L,respectively,which were significantly higher than the control group.The difference was statistically significant(P<0.05),and the difference between the three groups was statistically significant(P<0.05).Compared with the serum ADMA level,the light,moderate and severe damage groups were found to be (50.24±10.28)μmol/L,(74.72±11.84)μmol/L and (91.71±10.49)μmol/L were significantly higher than the control group,and there were significant differences between the two groups (P<0.05).Speaman rank correlation and Pearson correlation analysis showed that there was a positive correlation between the serological data of the two groups and the severity of neurological function related score in stroke patients.Namely,the more severe the neurological impairment was,the higher the serum PARK7 and ADMA levels were (rPARK7=0.996 7,PPARK7=0.003 3;rADMA=0.999 7,PADMA=0.000 3),the difference was statistically significant(P<0.05).Conclusion When a stroke accident occurs,it usually shows different degrees of neurological deficit.The serum levels of PARK7 and ADMA are also positively correlated,which may provide a theoretical basis for the early diagnosis and treatment of stroke,and the outcome of different degrees of stroke.Judgment has a certain guiding role,which is conducive to treatment and disease assessment.
【Key words】 Stroke;Human Parkinson's protein 7 (PARK7);DJ-1 protein;Asymmetric dimethylarginine (ADMA);Serum;Neurological deficit
脑卒中为神经系统常见病之一,现已高居全球死亡病因第2位[1],是致死率和致残率最高的神经系统疾病之一。因而发病早期准确的诊断对脑卒中的预防和诊断有重要意义,也是降低其致死率和致残率的方案之一[2]。虽然目前影像学检查包括颅脑CT、MRI等基本可确诊脑急性脑卒中,但还应注意在影像学不容易获取的环境中,亦或影像学检查不能很好地显示轻度脑缺血损伤的患者中,是否有生物标志物指标可能也是一种重要的诊断工具[3]。由于脑卒中存在个体异质性,因此需要更多可以早期判断脑缺血损伤的指标得以辅助诊断。随着近年来新的脑卒中生物标志物检测的引入,对于脑损伤的诊疗、病情评估和预后有重要指导意义[4]。研究发现,一些单独检测的标记物具有显著的敏感性和特异性[5-6]。但这些标志物大多在短期队列研究中进行,其出现在脑卒中发病后的血液中较晚,且血浆浓度无相对较高的敏感性和特异性,其检测指标提示脑损伤和脑卒中的能力相对较低。因而引入新的脑特异性标记物可使其更容易在血液样本中检测到,并可在卒中发生后较早提供诊断、治疗及预后等方面的预测依据。回顾多项研究可以发现,在急性卒中发生后,血浆中即可出现人帕金森蛋白7(PARK7)、非对称二甲基精氨酸(ADMA)水平变化,预示着其可作为中风早期诊断的血浆生物标志物之一[7-9]。因此对血清PRAK7、ADMA的研究或许可以为脑卒中早期诊断提供依据。本文选择82例损害不同程度的脑卒中患者,研究其血清PARK7、ADMA水平与神经功能缺损的相互关系。
1 资料与方法
1.1 临床资料 随机选取2017-01-01―2018-01-01至广西中医药大学附属瑞康医院治疗的脑卒中患者82例为实验组,同时随机选取非脑卒中患者82例为对照研究。入组标准:(1)脑卒中组患者症状判别依据“FAST”判断法[10]并及时送至医院;(2)经神经学体格检查、颅脑CT等明确诊断;(3)对照组排除神经系统疾病;(4)心电图、肝肾功能提示无明显异常;(5)无精神类疾病;(6)患者或其家属知情本研究并同意。本研究经广西中医药大学附属瑞康医院医学临床研究伦理委员会审查批准。2组一般资料差异无统计学意义(P<0.05),具有可比性。见表1。依据《脑卒中疾病神经功能相关缺损程度评分标准》[11-12]对脑卒中组患者进行量化评分,并以此分为轻度损害组48例、中度损害组20例及重度损害组14例。
表1 2组一般资料比较
Table 1 Comparison of general information of 2 groups
| 资料 |
脑卒中组(n=82) |
对照组(n=82) |
统计值 |
P值 |
| 性别比(男/女) |
56/26 |
51/31 |
0.6722 |
0.4123 |
| 年龄(x±s,岁) |
58.6±9.2 |
57.9±11.3 |
0.435 |
0.6641 |
| BMI(x±s,kg/m2) |
23.5±3.1 |
23.3±2.9 |
0.4266 |
0.6702 |
| 脑卒中类型[n(%)] |
|
|
|
|
| 脑梗死 |
49(59.76) |
52(63.41) |
0.232 |
0.6301 |
| 脑出血 |
33(40.24) |
30(36.59) |
0.232 |
0.6301 |
| 基础病因[n(%)] |
|
|
|
|
| 糖尿病 |
24(29.27) |
21(25.61) |
0.2756 |
0.5996 |
| 高血压 |
19(23.17) |
24(29.27) |
0.788 |
0.3747 |
| 高血脂 |
16(19.51) |
18(21.95) |
0.1484 |
0.7001 |
| 合并两种 |
|
|
|
|
| 或以上疾病 |
23(28.05) |
19(23.17) |
0.5121 |
0.4742 |
1.2 方法及观察指标 依据《脑卒中疾病神经功能相关缺损程度评分标准》对所有入院的脑卒中组患者进行量化评分,1~15分为轻度损害组,16~30分为中度损害组,31~45分为重度损害组。治疗前测定所有患者血清PARK7、ADMA水平,作为参考依据。
1.3 统计学处理 使用 SPSS 25.0软件进行统计学分析,计量资料采用均数±标准差(x±s)表示,两样本比较采用t检验;血清PARK7、ADMA水平变化与脑卒中神经功能缺损程度的互相关系运用Speaman等级相关和Pearson相关性分析,P<0.05为差异有统计学意义。
2 结果
2.1 各组神经功能相关评分及血清PARK7、ADMA水平情况比较 轻、中及重度损害组血清PARK7水平及ADMA水平均明显高于对照组,差异有统计学意义(P<0.05),且3组间对比差异也有统计学意义(P<0.05)。见表2。
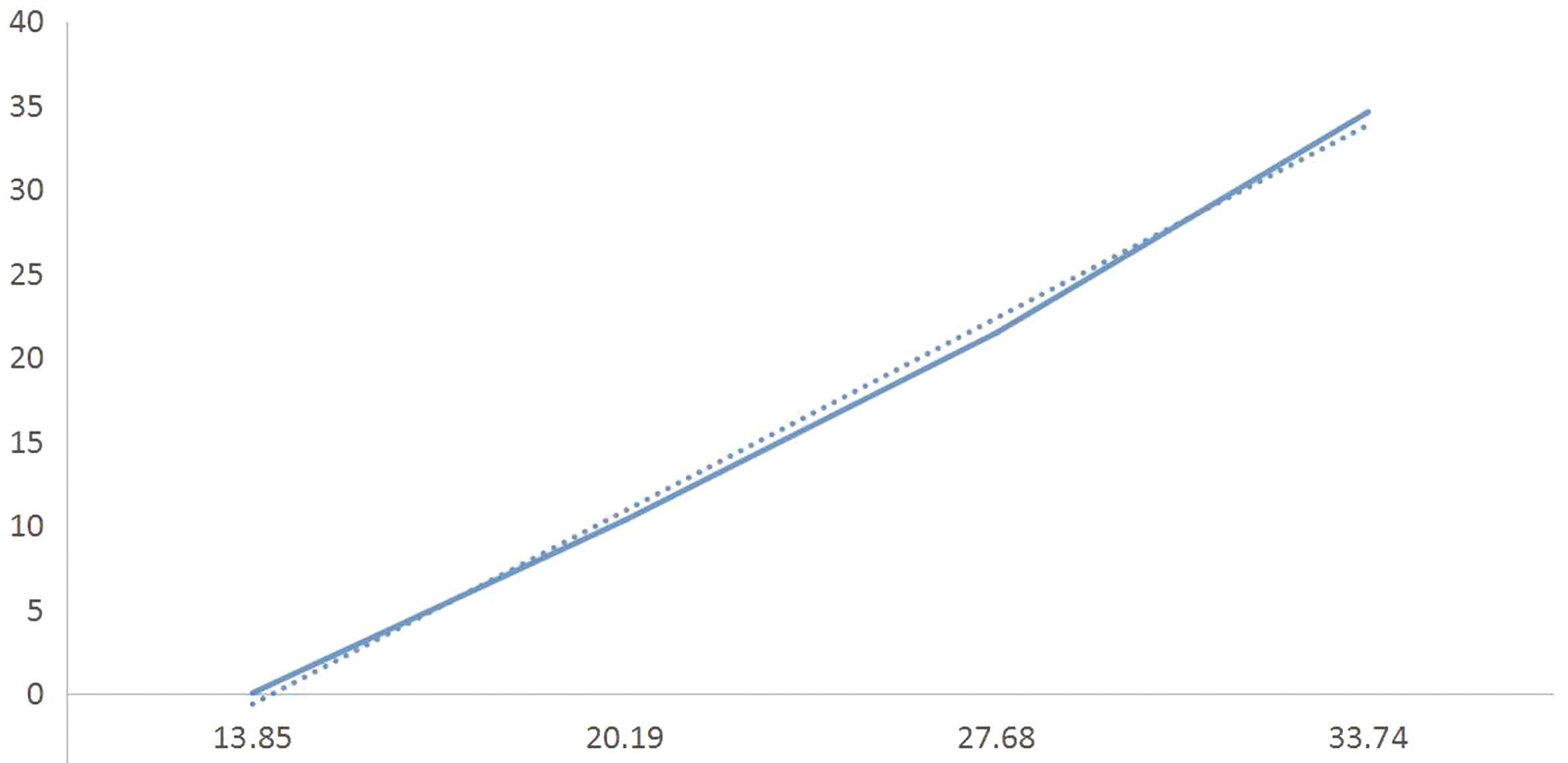
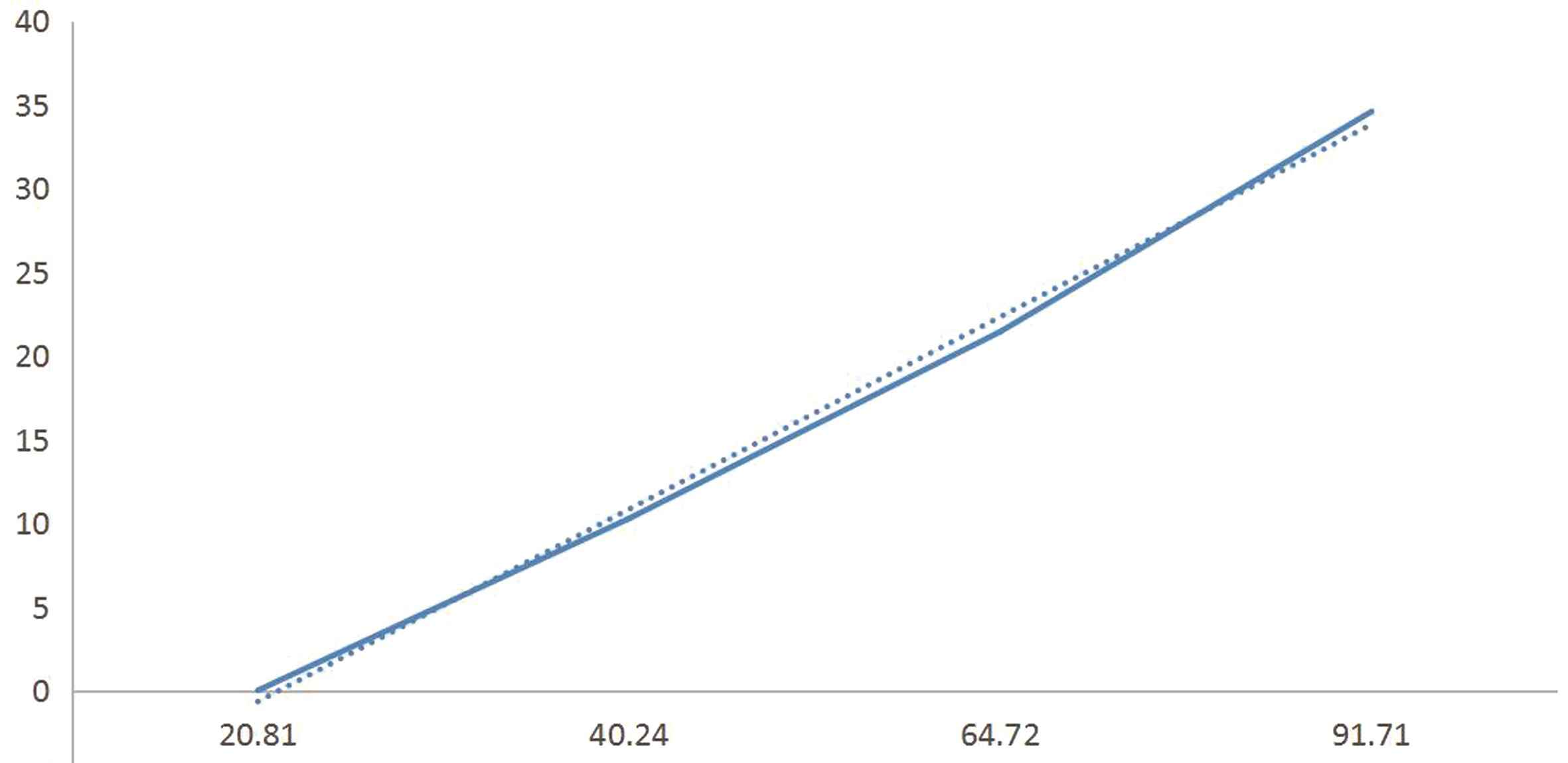
2.2 血清PARK7、ADMA水平变化与神经功能缺损程度相互关系 2组血清学数据使用Speaman等级相关和Pearson相关性分析发现,其水平变化与脑卒中患者神经功能相关评分严重程度呈正相关变化,即脑卒中病情越重,相应神经功能相关评分也越高,血清PARK7、ADMA水平越高(rPARK7=0.996 7,PPARK7=0.003 3;rADMA=0.999 7,PADMA=0.000 3),差异有统计学意义(P<0.05)。见图1、图2。
3 讨论
脑卒中分为脑梗死和脑出血,其中脑梗死约占70%,常见的卒中危险因素,如糖尿病、高血压、高血脂、心脏病、年龄及吸烟等均可引起脑血管内皮损伤[13]。研究表明,急性脑卒中事件发生后,血清PARK7及ADMA水平即出现变化,且随卒中严重程度呈时间型增加[7-9,14]。
表2 各组神经功能相关评分及血清PARK7、ADMA水平比较 (x±s)
Table 2 Comparison of neurological function-related scores and serum PARK7 and ADMA levels in each group (x±s)
| 组别 |
神经功能相关评分 |
PARK7(μg/L) |
ADMA(μmol/L) |
| 对照组 |
0.00 |
13.85±3.02 |
20.81±5.92 |
| 轻度损害组 |
10.27±4.161 |
20.19±4.211 |
40.24±10.281 |
| 中度损害组 |
21.43±5.6712 |
27.68±4.5312 |
64.72±11.8412 |
| 重度损害组 |
34.58±4.79123 |
33.74±5.27123 |
91.71±10.49123 |
| F值 |
158.04 |
177.94 |
375.24 |
| P值 |
<0.0001 |
<0.0001 |
<0.0001 |
注:与对照组比较,1P<0.05;与轻度损害组比较,2P<0.05;与中度损害组比较,3P<0.05;与重度损害组比较,4P<0.05
图1 血清PARK7水平变化与神经功能缺损程度的相互关系
Figure 1 Correlation between changes in serum PARK7 levels and degree of neurological deficits
图2 血清ADMA水平变化与神经功能缺损程度的相互关系
Figure 2 Correlation between changes in serum ADMA levels and degree of neurological deficits
血清PARK7在正常机体内含量较低,研究发现其在脑损害区域表达十分丰富。主因PARK7是一种关键的抗活性氧的氧化应激反应蛋白。可通过线粒体途径参与中风后的急性内源性神经保护,从而减少线粒体依赖的氧化应激导致细胞凋亡,从而对缺血/再灌注损伤引起的神经变性具有神经保护作用[7,15-17]。研究发现PARK7是与中风密切相关的生物标志物。卒中3 h内即可见PARK7浓度均高,其敏感性54%~91%,提示其可作为急性卒中早期诊断的可靠生物标志物[18]。PARK7在不同类型的卒中均可通过抗氧化作用防止细胞凋亡事件,但其具体机制仍然在研究中[19-21]。关于小鼠缺血性卒中模型诱导脑中动脉闭塞的实验发现,PARK7相关化合物可降低小鼠梗死体积,预防神经元死亡,从而减轻神经功能障碍[22-24]。因此可以预测,PARK7在缺血性卒中的早期诊断、对卒中后脑保护机制以及对卒中的治疗中可能都发挥着重要的作用。由于血清学改变往往均快于影像学的变化,在不能获取影像学检查或轻型卒中患者影像学无法辨别时,有效的生物学指标会更加敏感,对提高脑卒中的早期诊断率以及治疗方面可能起着十分重要的作用。
研究证实,诸多危险因素均可诱发一种因子产生过多ADMA[25-26]。ADMA是动脉粥样硬化和内皮细胞功能障碍的中介物质,属于内源性一氧化氮合酶抑制剂。研究发现ADMA可能在以某种特异性方式促进动脉粥样硬化进程[27]。多项研究也表明血浆ADMA水平在高胆固醇、高血压[28-29]、高同型半胱氨酸血症[30-31]、糖尿病[32]、年龄和吸烟[33]等血管性高危因素存在的患者中升高[34]。而这些高危因素恰恰也是脑血管事件的高危因素,可以预测ADMA与脑卒中的发生也存在某种关联。由此开展的临床研究发现,急性脑卒中事件发生后,ADMA水平随脑卒中严重程度的升高而升高,可能是通过减少脑血流、促进氧化应激和炎症反应而在脑损伤中发挥作用[9,35]。很多临床研究也探讨了血浆ADMA水变化与脑血管疾病的相关性,发现急性脑卒中后血浆ADMA浓度随脑卒中严重程度的增加呈时间型增加[36-39]。虽ADMA对卒中风险的作用愈发明显,但其在急性卒中损伤中的作用尚不清楚。研究表明,氧化应激引起的两种不同机制均可导致细胞内ADMA水平升高[40-41]。在卒中患者中ADMA的增加可能不仅是卒中后脑损伤的标志物,也是脑损伤的中介物质。它可通过抑制NOS对静息状态和急性卒中后脑血管顺应性以及限制脑血流发挥重要作用[42]。 ADMA对急性缺血性脑卒中的另一个影响可能是抑制nNOS NO的产生,保护神经元不受过度表达NO介导的损伤从而对中枢神经系统起到保护作用[43]。
基于上述研究,或许在卒中后早期ADMA浓度可以诊断和预测卒中结局,对脑卒中诊治及评估预后价值可能超越传统的危险因素,从而指导脑卒中预防的新方法[44-50]。血清PARK7、ADMA虽作用机制不同,但均在脑卒中发生时明显升高,且在卒中治疗、结局预测方面具有一定的相关性[51-56],为本研究提供充足了的理论依据。
本研究显示,卒中发生时血清PARK7可见升高,可能是其抗氧化、保护神经元等机制作用引起,同时ADMA水平亦增高,与ADMA减少脑血流、促氧化应激等作用机制相关。两者虽作用机制不同,但均在急性脑卒中发挥重要作用。另外,利用Speaman等级相关和Pearson相关性分析,发现2组血清学数据与脑卒中患者神经功能相关评分严重程度呈正相关变化。即脑卒中病变越重,相应神经功能相关评分也越高,血清PARK7、ADMA水平亦越高,提示可以通过这两个血清学分子提高脑卒中早期诊断及疗效的判定。
脑卒中发生时,患者常出现不同程度的神经功能缺损,而血清中PARK7、ADMA水平呈正相关变化,或许能为脑卒中的早期诊断及治疗提供新的血清生物学依据,也可对不同程度脑卒中转归的判断具有指导作用,有利于卒中治疗及病情评估,可以解决社会医疗的大难题。
4 参考文献
[1] 崔桂萍,刘萍.脑卒中患者血清PARK水平变化及意义[J].山东医药,2013,53(24):12-14.
[2] AGEMANG C,VAN OEFFELEN A A,NORREDAM M,et al.Ethnic disparities in ischemic stroke,intracerebral hemorrhage,and subarachnoid hemorrhage incidence in the Netherlands[J].Stroke,2014,45(11):3 236-3 242.
[3] JICKLING G C,SHARP F R.Biomarker panels in ischemic stroke[J].Stroke,2015,46(3):915-920.
[4] LI J,WANG Y.Blood Biomarkers in Minor Stroke and Transient Ischemic Attack [J].Neurosci Bull,2016,32(5):463-468.
[5] BUSTAMANTE A,LOPEZ-CANZIO E,PICH S,et al.Blood biomarkers for the Early Diagnosis of Stroke:The Stroke-Chip Study[J].Stroke,2017 Sep,48(9):2 419-2 425.
[6] REYNOLDS M A,KIRCHICK H J,DAHLEN J R,et al.Early biomarkers of stroke[J].Clin Chem,2003,49(10):1 733-1 739.
[7] ALLARD L,BURKHARD P R,LESCUYER P,et al.PARK7 and nucleoside diphosphate kinase A as plasma markers for the early diagnosis of stroke[J].ClinChem,2005,51(11):2 043-2 051.
[8] KANEKO Y,TAJIRI N,SHOJO H,et al.Oxygen-glucose-deprived rat primary neural cells exhibit DJ-1 translocation into healthy mitochondria:a potent stroke therapeutic target[J].CNS Neurosci Ther,2014,20(3):275-281.
[9] WORTHMANN H,CHEN S,MARTENS-LOBENHOFFER J,et al.High plasma dimethylarginine levels are associated with adverse clinical outcome after stroke[J].J Atheroscler Thromb,2011,18(9):753-761.
[10] 章立,蔡海波,金友雨.非对称性二甲基精氨酸在急性缺血性脑卒中患者的研究[J].中国临床药理学杂志,2013,29(8):595-597.
[11] LANGEN J,KAYACELEBI A A,BECKMANN B,et al.Homoarginine (hArg) and asymmetric dimethylarginine (ADMA) in short stature children without and with growth hormone deficiency:hArg and ADMA are involved differently in growth in the childhood[J].Amino Acids,2015,47(9):1 875-1 883.
[12] 王继贵.脑卒中新的生物标志物研究进展[J].实验与检验医学,2012,30(4):323-327.
[13] 佘瑞芳,伏兵,刘建华等.血浆ADMA水平与急性脑梗死的相关性研究[J].重庆医学,2013,25(30):3 601-3 603.
[14] CHEN S,LI N,DEB-CHATTER JI M,et al.Asymmetric dimethyarginine as marker and mediator in ischemic stroke[J].Int J Mol Sci,2012,13(12):15 983-16 004.
[15] MINGINA T,ZHAO M.Role of PARK7 and NDKA in stroke management:a review of PARK7 and NDKA as stroke biomarkers[J].Biomark Med,2018,12(5):419-425.
[16] ALEYASIN H,ROUSSEAUX M W,PHIILLIPS M,et al.The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage[J].Proc Natl Acad Sci USA,2007,104(47):18 748-18 753.
[17] HARSTON GW,RANE N,SHAYA G,et al.Imaging biomarkers in acute ischemic stroke trials:a systematic review[J].Am J Neuroradiol,2015,36(5):839-843.
[18] ZONDLER L,MILLER-FLEMING L,REPICI M,et al.DJ-1 interactions with α-synuclein attenuate aggregation and cellular toxicity in models of Parkinson's disease[J].Cell Death Dis,2014,5:e1350.
[19] KIM T,VEMUGANTI R.Mechanisms of Parkinson's disease-related proteins in mediating secondary brain damage after cerebral ischemia[J].J Cereb Blood Flow Metab,2017,37(6):1 910-1 926.
[20] HAN X,GAO Y,MA B,et al.The Clinical Relevance of Serum NDKA,NMDA,PARK7,and UFDP Levels with Phlegm-Heat Syndrome and Treatment Efficacy Evaluation of Traditional Chinese Medicine in Acute Ischemic Stroke[J].Evid Based Complement Alternat Med,2015,2015:270 498.
[21] ZIMMERMANN-IVOL C G,BURKHARD P R,LE FLOCH-ROHR J,et al.Fatty acid binding protein as a serum marker for the early diagnosis of stroke:a pilot study[J].Mol Cell Proteomics,2004,3(1):66-72.
[22] HIJIOKA M,INDEN M,YANAGISAWA D,et al.DJ-1/PARK7:A New Therapeutic Target for Neurodegenerative Disorders[J].Biol Pharm Bull,2017,40(5):548-552.
[23] ARIGA H,TAKAHASHI-NIKI K,KATO I,et al.Neuroprotective function of DJ-1 in Parkinson's disease[J].Oxid Med Cell Longev,2013,2013:683 920.
[24] YANAGIDA T,KITAMURA Y,YAMANE K,et al.Protection against oxidative stress-induced neurodegeneration by a modulator for DJ-1,the wild-type of familial Parkinson's disease-linked PARK7[J].J Pharmacol Sci,2009,109(3):463-468.
[25] 王建秋,卜培莉.非对称性二甲基精氨酸与颈动脉硬化的关系[J].中国老年学杂志,2013,33(23):5 805-5 806.
[26] 张德永,袁惠清,何洪怀,等.急性脑梗死患者血浆ADMA与血清Hcy的相关性[J].西部医学,2015,27(3):412-414;417.
[27] MAAS R,XANTHAKIS V,POLAK J F,et al.Association of the endogenous nitric oxide synthase inhibitor ADMA with carotid artery intimal media thickness in the Framingham Heart Study offspring cohort[J].Stroke,2009,40(8):2715-2719.
[28] KIELSTEIN J T,BODE-BOGER S M,FROLICH J C,et al.Asymmetric dimethylarginine,blood pressure,and renal perfusion in elderly subjects[J].Circulation,2003,107(14):1 891-1 895.
[29] NISHIYAMA Y,UEDA M,KATSURA K,et al.Asymmetric dimethylarginine (ADMA) as a possible risk marker for ischemic stroke[J].J Neurol Sci,2010,290(1/2):12-15.
[30] BOGER R H,LENTA S R,BODE-BOGER S M,et al.Elevation of asymmetrical dimethylarginine may mediate endothelial dysfunction during experimental hyperhomocyst(e)inaemia in humans[J].Clin Sci,2001,100(2):161-167.
[31] STUHLINGER M C,STANGER O.Asymmetric dimethyl-L-arginine (ADMA):a possible link between homocyst(e)ine and endothelial dysfunction[J].Curr Drug Metab,2005,6(1):3-14.
[32] ABBASI F,ASAGMI T,COOKE J P,et al.Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus[J].Am J Cardiol.2001,88(10):1 201-1 203.
[33] JUONALA M,VIIKARI J S,ALFTHAN G,et al.Brachial artery flow-mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in young Finns study[J].Circulation,2007,116(12):1 367-1 373.
[34] MOLNAR T,PUSCH G,NAGY L,et al.Correlation of the L-Arginine Pathway with Thrombo-Inflamma-tion May Contribute to the Outcome of Acute Ischemic Stroke[J].J Stroke Cerebrovasc Dis,2016,25(8):2 055-2 060.
[35] PIKULA A,BOGER R H,BEISER A S,et al.Association of plasma ADMA levels with MRI markers of vascular brain injury:Framingham offspring study[J].Stroke,2009,40(9):2 959-2 964.
[36] YOO J H,LEE S C.Elevated levels of plasma homocyst(e)ine and asymmetric dimethylarginine in elderly patients with stroke[J].Atherosclerosis,2001,158(2):425-430.
[37] NOTSU Y,NABIKA T,BOKURA H,et al.Evaluation of asymmetric dimethylarginine and homocysteine in microangiopathy-related cerebral damage[J].Am J Hypertens,2009,22(3):257-262.
[38] KHAN U,HASSAN A,VALLANCE P,et al.Asymmetric dimethylarginine in cerebral small vessel disease[J].Stroke,2007,38(2):411-413.
[39] BROUNS R,MARESCAU B,POSSEMIERS I,et al.Dimethylarginine levels in cerebrospinal fluid of hyperacute ischemic stroke patients are associated with stroke severity[J].Neurochem Res,2009,34(9):1 642-1 649.
[40] LEIPER J,MURRAY-RUST J,MCDONALD N,et al.S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity:further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase[J].Proc Natl Acad Sci USA,2002,99(21):13 527-13 532.
[41] NAIR N,GONGORA E.Oxidative Stress and Cardiovascular Aging:Interaction Between NRF-2 and ADMA[J].Curr Cardiol Rev,2017,13(3):183-188.
[42] IADECOLA C,PELLIGRINO D A,MOSKOWITZ M A,et al.Nitric oxide synthase inhibition and cerebrovascular regulation[J].J Cereb Blood Flow Metab,1994,14(2):175-192.
[43] CARDOUNEL A J,ZWEIER J L.Endogenous methylarginines regulate neuronal nitric-oxide synthase and prevent excitotoxic injury[J].J Biol Chem,2002,277(37):33 995-34 002.
[44] SCHERBAKOV N,SANDEK A,MARTENS-LOBENHOFFER J,et al.Endothelial dysfunction of the peripheral vascular bed in the acute phase after ischemic stroke[J].Cerebrovasc Dis,2012,33:37-46.
[45] WANBY P,TEERLINK T,BRUDIN L,et al.Asymmetric dimethylarginine (ADMA) as a risk marker for stroke and TIA in a Swedish population[J].Atherosclerosis,2006,185(2):271-277.
[46] HALAI A D,WOOLLAMS A M,LAMBON RALPH M A.Triangulation of language-cognitive impairments,naming errors and their neural bases post-stroke[J].Neuroimage Clin,2017,17:465-473.DOI:10.1016/j.nicl.2017.10.037.
[47] WILLIAMS T S,MCDONALD K P,ROBERTS S D,et al.Prevalence and Predictors of Learning and Psychological Diagnoses Following Pediatric Arterial Ischemic Stroke[J].Dev Neuropsychol,2017,42(5):309-322.DOI:10.1080/87565641.2017.1353093.
[48] THOMSEN B,GAROSI L,SKERRITT G,et al.Neurological signs in 23 dogs with suspected rostral cerebellar ischaemic stroke[J].Acta Vet Scand,2016,58(1):40.DOI:10.1186/s13028-016-0219-2.
[49] GUILLAN M,DEFELIPE-MIMBRERA A,ALONSO-CANOVAS A,et al.The syndrome of transient headache and neurological deficits with cerebrospinal fluid lymphocytosis mimicking an acute stroke[J].Eur J Neurol,2016,23(7):1235-1240.DOI:10.1111/ene.13008.E
[50] KESSNER S S,BINGEL U,THOMALLA G.Somatosensory deficits after stroke:a scoping review[J].Top Stroke Rehabil,2016,23(2):136-146.DOI:10.1080/10749357.2015.1116822.
[51] MIMS K N,KIRSCH D.Sleep and Stroke[J].Sleep Med Clin,2016,11(1):39-51.DOI:10.1016/j.jsmc.2015.10.009.
[52] NIWA S,SHIMODOZONO M,KAWAHIRA K.Prevalence and association of visual functional deficits with lesion characteristics and functional neurological deficits in patients with stroke[J].Neuro Rehabilita-tion,2015,37(2):203-211.DOI:10.3233/NRE-151253.
[53] SEMRAU J A,WANG J C,HERTER T M,et al.Relationship between visuospatial neglect and kinesthetic deficits after stroke[J].Neurorehabil Neural Repair,2015,29(4):318-328.DOI:10.1177/1545968314545173.
[54] KOTLEGA D,NOWACKI P,BIAECKA M,et al.Association between CRP gene polymorphism 717A/G,C-reactive protein and neurological deficit in ischemic stroke[J].J Clin Neurosci,2014,21(4):574-577.DOI:10.1016/j.jocn.2013.06.016.
[55] XIN X Y,GAO Y.The use of repeated measures analysis of variance to study the effect of phlegm-heat syndrome on neurological deficits in patients with stroke[J].Chin J Integr Med,2013,19(8):568-572.DOI:10.1007/s11655-012-1087-x.
[56] KRAUSE T,NOLTE C H.The syndrome of transient headache and neurological deficits with cerebrospinal fluid lymphocytosis (HaNDL) as an acute ischemic stroke mimic leading to systemic thrombolysis:a case report[J].Clin Neurol Neurosurg,2012,114(6):689-690.DOI:10.1016/j.clineuro.2011.11.017.
(收稿2019-01-05)
本文责编:张喜民
本文引用信息:曹康,张永全,蔡碧清,胡洒洒,潘珠娣.脑卒中患者血清PARK7及ADMA水平与神经功能缺损的关系[J].中国实用神经疾病杂志,2019,22(3):260-266.DOI:10.12083/SYSJ.2019.03.050
Reference information:CAO Kang,ZHANG Yongquan,CAI Biqing,HU Sasa,PAN Zhudi.Relationship between serum PARK7,ADMA and neurological deficit in stroke patients[J].Chinese Journal of Practical Nervous Diseases,2019,22(3):260-266.DOI:10.12083/SYSJ.2019.03.050