目的 探讨磁共振成像表观弥散系数(ADC)值及动脉自旋标记成像脑血流量(CBF)值在大面积急性缺血性脑卒中出血转化(HT)预测中的临床应用。方法 收集研究2016-01—2018-06经临床和影像学证实的60例急性期大面积缺血性脑卒中患者(HT组35例,无HT组25例),采用GE 3.0T MRI进行常规序列和3D-PCASL检查,通过软件处理后获取梗死核心区表观弥散系数(ADC)图和CBF伪彩图,分析HT与梗死核心区ADC值的相关性,同时依据CBF伪彩图提示梗死核心区是否出现点状或条片状高灌注信号分
表观弥散系数及脑血流量值在急性大面积缺血性脑卒中出血转化预测中的应用价值
卢明聪1) 符大勇1) 孟 云1) 周建国1)△ 马先军2) 刘晓丽2)
南京中医药大学连云港附属医院 1)放射科 2)脑病科,江苏 连云港 222004
基金项目:南京医科大学康达学院2018年度科研发展基金课题(编号:KD2018KYJJYB027)
作者简介:卢明聪,Email:lygzyylmc@163.com
△通信作者:周建国,Email:13645132158@163.com
【摘要】 目的 探讨磁共振成像表观弥散系数(ADC)值及动脉自旋标记成像脑血流量(CBF)值在大面积急性缺血性脑卒中出血转化(HT)预测中的临床应用。方法 收集研究2016-01—2018-06经临床和影像学证实的60例急性期大面积缺血性脑卒中患者(HT组35例,无HT组25例),采用GE 3.0T MRI进行常规序列和3D-PCASL检查,通过软件处理后获取梗死核心区表观弥散系数(ADC)图和CBF伪彩图,分析HT与梗死核心区ADC值的相关性,同时依据CBF伪彩图提示梗死核心区是否出现点状或条片状高灌注信号分为高灌注组和低灌注组,评估梗死核心区不同灌注状态及CBF值与HT发生的相关性。结果 入组患者60例,其中HT患者35例,HT转化组平均ADC值为(0.49±0.18)×10-3mm2/s,低于无HT组,梗死区高灌注组平均CBF值大于低灌注组,高灌注组HT发生率为80.76%,高灌注组出血转化率明显高于低灌注组。结论 急性期大面积梗死区ADC值、梗死区高灌注是缺血性脑卒中出血转化的独立危险因素,与HT发生显著相关,对于临床制定合理治疗方案及预后评估具有重要价值。
【关键词】 缺血性脑卒中;出血转化;磁共振;表观弥散系数;动脉自旋标记
【中图分类号】 R743.3 【文献标识码】 A 【文章编号】 1673-5110(2018)20-2205-07 DOI:10.12083/SYSJ.2018.20.477
Value of apparent diffusion coefficient and cerebral blood flowin predicting hemorrhagic transformation in acute massive ischemic stroke
LU Mingcong1),FU Dayong1),MENG Yun1),ZHOU Jianguo1),MA Xianjun2),LIU XiaoLi2)
1)Department of Radiology,Lianyungang TCM Hospital Affiliated to Nanjing University of Chinese Medicine,Lianyungang 222004,China;2)Department of Encephalopathy,Lianyungang TCM Hospital Affiliated to Nanjing University of Chinese Medicine,Lianyungang 222004,China
【Abstract】 Objective To explore the clinical application of apparent diffusion coefficient (ADC) and cerebral blood flow (CBF) value of magnetic resonance imaging in predicting hemorrhagic transformation (HT) of acute ischemic stroke in large area.Methods 60 cases (35 patients in the HT transformation group and 25 patients in the non-bleeding group) of acute cerebral apoplexy confirmed by clinical and imaging in from January 2016 to June 2018 were collected,and the GE 3.0T MRI was used for routine sequence and 3D-PCASL examination,and the apparent diffusion coefficient (ADC) and CBF pseudo color map of the infarct core area were obtained after the software treatment.To analyze the correlation between HT and ADC value of infarct core area,and to divide the infarct core area into hyperperfusion group and hypoperfusion group according to whether dot or strip hyperperfusion signal appears in CBF pseudocolor image,and to evaluate the correlation between different perfusion status of infarct core area and CBF value and HT occurrence.Results There were 60 patients in the group,of which 35 cases were HT,the ADC value of the HT transformation group was (0.49±0.18)×10-3mm2/s,lower than that without HT,the CBF value in the high perfusion group was greater than that in the low perfusion group,and the incidence of HT in the high perfusion group was 80.76%,and the hemorrhage transformation rate in the high perfusion group was significantly higher than that in the low perfusion group.Conclusion ADC and high perfusion in acute infarct area are the independent risk factors of hemorrhagic transformation in ischemic stroke.It has significant statistical significance with the occurrence of HT.It is of great value for the development of rational treatment plan and prognosis evaluation.
【Key words】 Ischemic stroke;Hemorrhagic transformation;Magnetic resonance;Apparent diffusion coefficient;Arterial spin labeling
缺血性脑卒中为我国最常见的脑卒中类型,约占3/4。其发生机制为供血动脉的狭窄、严重闭塞导致脑组织的血供减少或停滞,导致脑实质缺血缺氧,进而发生软化[1-4]。目前,急性期治疗多采用溶栓、血管内治疗以及抗血小板治疗等,但急性脑梗死出血转化(hemorrhagic transformation,HT)的发生直接影响临床治疗的有效性和安全性。本研究通过量化的表现弥散系数(apparent diffusion coefficient,ADC)及全脑血流量(cerebral blood flow,CBF)值预测大面积急性脑梗死发生出血转化的可能性,有利于临床采取个性化、精准化治疗方案。
1 资料与方法
1.1 一般资料 选取2016-01—2018-06在南京中医药大学连云港附属医院经临床和影像学证实的60例急性期缺血性脑卒中患者,男38例,女22例,年龄42~86岁;临床症状:偏身麻木、肢体不利、头晕、头痛及言语不清等。
1.2 纳入及排除标准 纳入标准:(1)入组患者均符合2018年《中国急性缺血性脑卒中诊治指南》诊断标准;(2)患者行首次MR检查时间为发病72 h以内;(3)脑梗死面积≥5 cm2;(4)首次MRI检查未见脑出血,2周内复查MRI或CT提示HT。排除标准:(1)脑干或基底节区腔隙性梗死;(2)由于外伤、肿瘤、血管畸形及凝血功能异常导致的颅内出血。
1.3 仪器与方法 使用GE 3.0T 750 磁共振扫描仪进行检查,8通道相控头颈线圈。对所有患者行轴位T1WI、T2WI、T2 FLAIR、DWI、SWAN、ASL检查。DWI参数:TR/TE=6 000 ms/73.5 ms;3D-ASL参数:TR/TE=5 369 ms/10.5 ms,FOV24 cm×24 cm,分辨率512×8,NEX3次,标记后延迟时间2 500 ms;SWAN参数:TR/TE=37.4 ms/22.9 ms,层厚2 mm,NEX0.70次,带宽62.5 kHz,反转角20°。
1.4 数据处理和分析 将原始数据传输至GE AW4.6工作站,使用Functool软件,通过ADC伪彩图选取感兴趣区(regions of interest,ROI),并测量ADC值,每个ROI随机测量3次、取其平均值。分析量化的ADC值与HT的相关性。将3D-ASL原始数据信息经Functool软件处理后得出全脑血流量(CBF)伪彩图,依据CBF伪彩图提示梗死核心区是否出现点状或条片状高灌注信号分为高灌注组和低灌注组,结合DWI图像,观察梗死核心区异常灌注信号,当发现高灌注信号时测量其CBF值,无高灌注信号则随机测量低灌注区,对每处感兴趣区重复测量3次,取其平均值[5-8],评估梗死核心区不同灌注状态、CBF值与HT发生的相关性。依据SWAN序列显示梗死核心区是否出现点状或斑片状低信号作为判定HT标准,按欧洲急性卒中合作研究(ECASS)诊断标准,通过CT或MRI SWAN序列检查将HT分为:(1)出血性梗死(hemorrhagic infarction,HI),包含梗死核心及周围无占位效应的斑点状出血。(2)实质血肿(parenehymal hemorrhage,PH),包含出血少、出血面积<梗死面积30%的PH1型;出血面积>梗死核心30%且有占位效应或远离梗死核心的出血,即PH2型[9-12]。
1.5 统计学分析 采用SPSS 22.0统计软件包进行数据分析,计量资料以均数±标准差(x±s)表示,经检验符合正态分布,使用独立样本t检验;计数资料以百分比(%)表示,采用χ2检验,P<0.05为差异有统计学意义。
2 结果
入组患者60例,其中HT患者35例,HT转化组ADC值低于无HT组(见表1);梗死核心区高灌注出血转化率明显高于低灌注组。见表2、表3。
表1 梗死核心区ADC值与出血转化发生率比较 (x±s)
Table 1 Comparison of ADC values and incidence ofhemorrhagic transformation in infarct core (x±s)
| 组别 |
n |
ADC值(×10-3mm2/s) |
t值 |
P值 |
| HT组 |
35 |
0.49±0.18 |
-2.723 9 |
0.008 5 |
| 无HT组 |
25 |
0.61±0.15 |
|
|
表2 梗死核心区灌注类型与出血转化发生率比较 (n)
Table 2 Comparison of perfusion type in infarctcore area and incidence of hemorrhagic transformation (n)
| 因素 |
PH型 |
HI型 |
未出血 |
总计 |
χ2 值 |
P值 |
| 高灌注 |
12 |
9 |
5 |
26 |
12.203 |
0.002 |
| 低灌注 |
4 |
10 |
20 |
34 |
|
|
表3 梗死核心区不同灌注类型CBF值与出血转化发生率比较 (x±s)
Table 3 Comparison of CBF values and incidence of hemorrhagictransformation in different perfusion types in the infarct core (x±s)
| 组别 |
CBF值[mL/(100 g×min)] |
| PH型 |
HI型 |
无HT |
| 高灌注组 |
70.4±13.5 |
66.5±12.7 |
52.6±15.2 |
| 低灌注组 |
38.7±11.7 |
22.6±8.4 |
20.9± 4.8 |
| t值 |
4.180 1 |
6.384 3 |
4.020 8 |
| P值 |
0.000 9 |
<0.000 1 |
0.001 3 |
3 讨论
3.1 大面积急性脑梗死出血转化发病机制 HT是在脑组织缺血、缺氧和坏死的基础上继发的出血。其发病机制包括:(1)梗死区血管壁内皮细胞的变性坏死导致血管通透性增加,导致红细胞外渗;(2)闭塞血管自发或治疗后再通引起的再灌注损伤;(3)梗死灶周围反应性水肿导致毛细血管受压,待水肿消退后侧支循环开放,毛细血管的缺血损伤容易发生破裂,引发HT[13-16]。既往研究发现,脑梗死后出血转化与梗死范围有一定相关性,梗死核心与HT发生呈正相关[17-20],少量出血相对于临床治疗所产生的影响较小,但随着出血量的增加加大了临床不良预后的发生概率。既往研究[8]发现,非血肿型HT患者的临床疗效优于血肿型HT,且治疗后NIHSS评分低于血肿型HT。
3.2 梗死核心区ADC值与HT发生的相关性 弥散成像(diffusion weighted imaging,DWI)是一种基于水分子布朗运动的成像方法[21-24],梗死核心区脑细胞肿胀限制了细胞外水分子的运动,在DWI上呈高信号,我们将其定义为梗死核心区(图1~2)。同时可以通过表观弥散系数(ADC)图像量化弥散受限程度,ADC值下降程度和细胞毒性水肿呈正相关。本研究中HT转化组ADC值低于无HT组(P<0.05),提示ADC值间接反映梗死区域神经组织缺血坏死程度,血脑屏障破坏越明显,HT的发生可能性增加。
3.3 缺血梗死区灌注状态与HT发生的相关性 ASL成像技术通过利用自体动脉血中的水分子作为内源性对比剂,利用反转脉冲于成像平面上游将其标记,通过将信号标记图像减影没有标记图像,得出灌注图像[25-28]。通过伪彩区分不同脑组织血液灌注状态,观察更为直接。因为标记的是动脉血中的水质子,ASL可通过测定CBF值量化单位时间内流经一定量脑组织血液(图1~2)。WANG等[12]研究证实,梗死核心区血脑屏障的破坏、侧支循环开放以及责任血管的再通可引发高灌注,当梗死核心区及周围高灌注时提示缺血梗死区动脉血液供应的增加,CBF伪彩图显示为斑点状、团片状、条状或不规则片状高灌注信号,提示HT发生概率增加,特别是梗死核心区出现明显高灌注时。本研究中梗死区高灌注组CBF值大于低灌注组,梗死区高灌注组HT发生率明显高于低灌注组(P<0.05)。
HT既是急性脑梗死的自然转归,也是最常见的并发症之一,微出血可能对于患者的治疗及预后影响较小,但是随着出血量的增加,可能会加重患者的病情,增加患者的致死致残率[29-33]。通过梗死核心区ADC值及CBF值的测量可以为HT的发生提供监测,能够为临床医师提供HT风险预测,这对于临床治疗方案的选择及预后评估具有重要临床应用价值[34-38]。
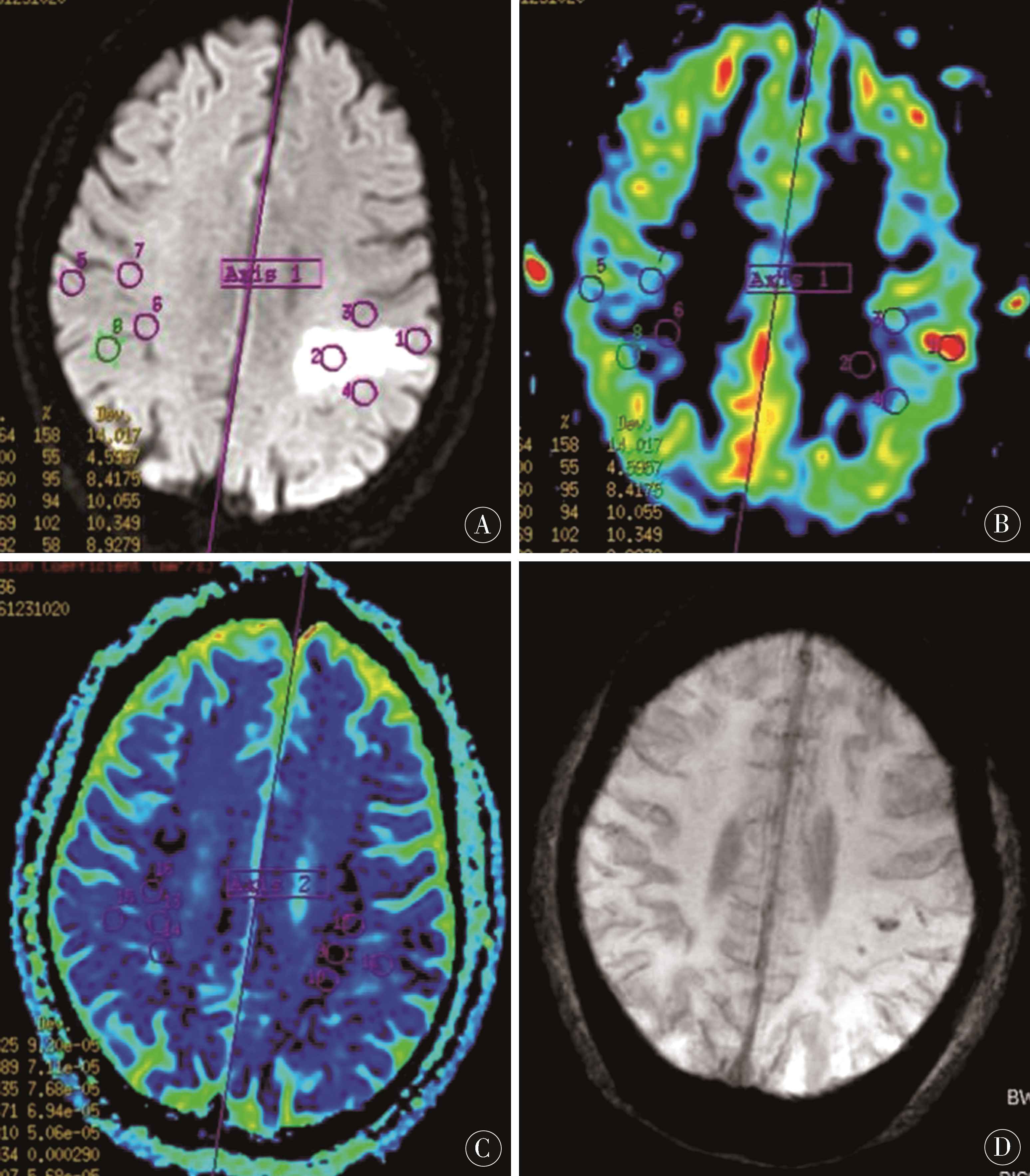
图1 A~D:为同一病人,女,80岁,因言语不利1 d入院,A:DWI像示左顶叶急性脑梗死;B:ASL像示左顶叶病灶CBF值35.6~44.1 mL/(100 g×min),较右侧降低,局部见点状高灌注,CBF值达97.5 mL/(100 g×min);C:ADC像左顶叶病灶ADC值0.63~0.66(10-3mm2/s);D:SWI像示病灶内点状HT
Figure 1 A-D:is the same patient,female,80 years old,hospitalized for 1 day because of speech impairment.A:DWI shows acute cerebral infarction in left parietal lobe;B:ASL shows CBF value of left parietal lobe lesion 35.6-44.1 mL/(100 g×min),which is lower than that of right side,with spot-like high perfusion and CBF value of 97.5 mL/(100 g×min);C:ADC shows ADC value of left parietal lobe lesion 0.63-0.66 (10-3 mm2/s);D:SWI shows dot-like HT in the lesion
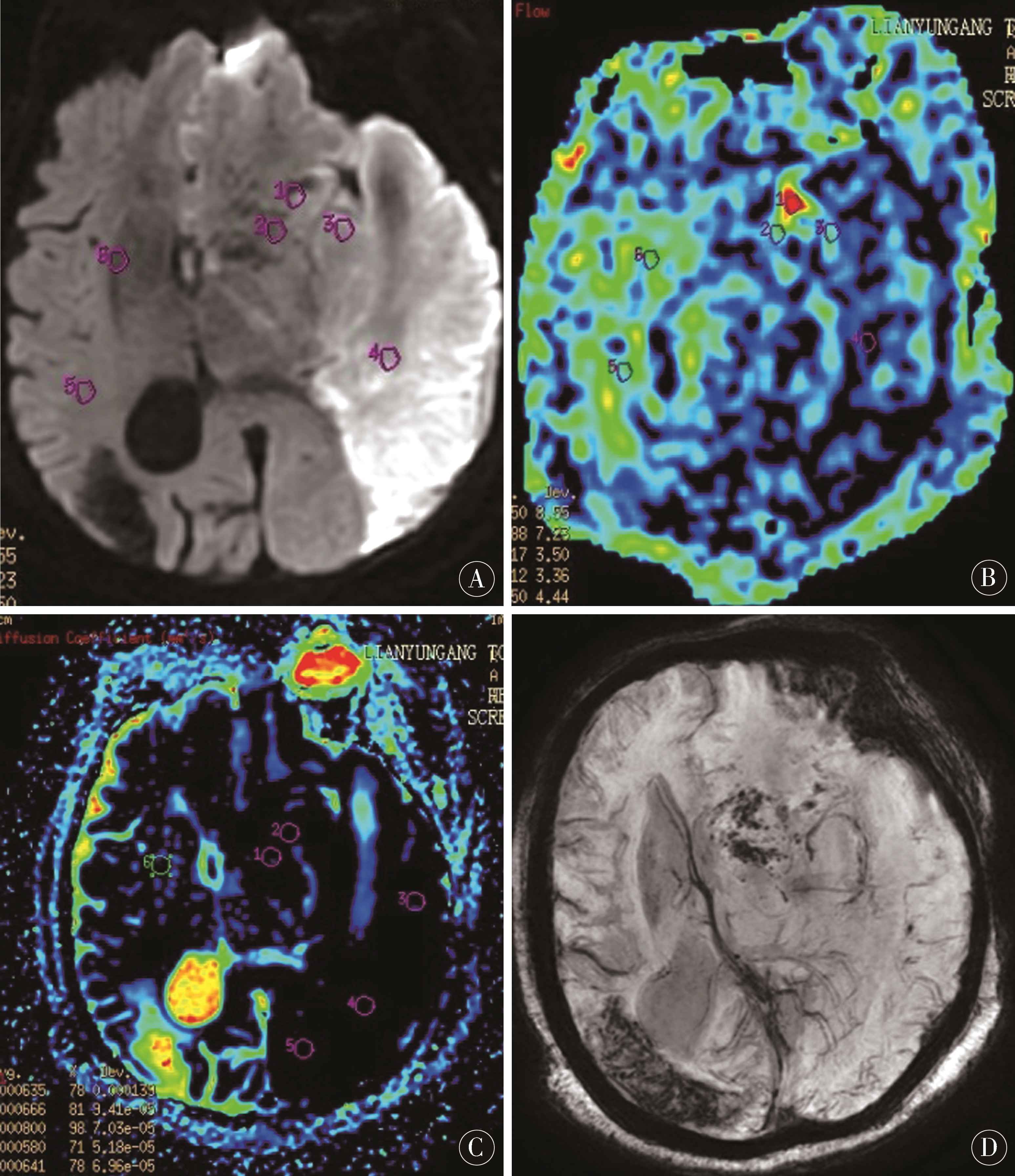
图2 A-D为同一病人,女,85岁,因右侧肢体活动不利3 h入院,A:DWI像示左额颞枕顶叶急性脑梗死;B:ASL像示病灶CBF值12.8~29.6 mL/(100 g×min),较右侧降低,边缘见点状高灌注,CBF值达66.2 mL/(100 g×min);C:ADC像病灶ADC值0.38~0.66(10-3mm2/s);D:SWI像示病灶边缘多发点状HT
Figure 2 A-D:was the same patient,female,85 years old,who was hospitalized for 3 hours because of poor right limb movement.A:DWI showed acute cerebral infarction in left frontotemporal-parietal lobe;B:ASL showed CBF value of 12.8-29.6 mL/(100 g×min),which was lower than that of the right side,with dot-like high perfusion at the edge and CBF value of 66.2 mL/(100 g×min);C:ADC showed ADC value of 0.38-0.66 (10-3 mm2/s);D:SWI showed multiple spots HT on the edge of the lesion
4 参考文献
[1] LI X, WANG Y,WANG Z,et al.Comparison of magnetic resonance spectroscopy (MRS) with arterial spin labeling (ASL) in the differentiation between mitochondrial encephalomyopathy,lactic Acidosis,plus stroke-like episodes (MELAS) and acute ischemic stroke (AIS)[J].J Clin Neurosci,2018,55:65-70.
[2] 冯跃明,杨辉.急性缺血性脑卒中患者静脉溶栓后不同部位出血转化的影响因素[J].中国实用神经疾病杂志,2016,19(3):59-61.
[3] NELSON M D.Left ventricular diastolic dysfunction in women with nonobstructive ischemic heart disease:insights from magnetic resonance imaging and spectros-copy[J].Am J Physiol Regul Integr Comp Physiol,2017,313(4):R322-R329.
[4] VON KUMMER R,DZIALOWSKI I.Imaging of cerebr-al ischemic edema and neuronal death[J].Neuroradiolo-gy,2017,59(6):545-553.
[5] LIBERMAN A L,KALANI R E,AW-ZORETIC J,et al.Cardiac magnetic resonance imaging has limited additional yield in cryptogenic stroke evaluation after transesophageal echocardiography[J].Int J Stroke,2017,12(9):946-952.
[6] JIMÉNEZ-XARRIÉ E,DAVILA M,CANDIOTA A P,et al.Brain metabolic pattern analysis using a magnetic resonance spectra classification software in experimental stroke[J].BMC Neurosci,2017,18(1):13.
[7] TUOR U I,QIAO M.Magnetic resonance imaging detection of multiple ischemic injury produced in an adult rat model of minor stroke followed by mild transient cerebral ischemia[J].MAGMA,2017,30(2):175-188.
[8] YANEV P,SEEVINCK P R,RUDRAPATNA U S,et al.Magnetic resonance imaging of local and remote vascular remodelling after experimental stroke[J].J Cereb Blood Flow Metab,2017,37(8):2 768-2 779.
[9] KOHNO N,OKADA K,YAMAGATA S,et al.Distinctive Patterns of Three-Dimensional Arterial Spin-Labeled Perfusion Magnetic Resonance Imaging in Subtypes of Acute Ischemic Stroke[J].J Stroke Cerebrovasc Dis,2016,25(7):1 807-1 812.
[10] MCGARRY B L,ROGERS H J,KNIGHT M J,et al.Stroke onset time estimation from multispectral quantitative magnetic resonance imaging in a rat model of focal permanent cerebral ischemia[J].Int J Strok,2016,11(6):677-682.
[11] YASSI N,CAMPBELL B C,MOFFAT B A,et al.Association between baseline peri-infarct magnetic resonance spectroscopy and regional white matter atrophy after stroke[J].Neuroradiology,2016,58(1):3-10.
[12] WANG D J,ALGER J R,QIAO J X,et al.The value of arterial spin-labeled perfusion imaging inacute ischemic stroke:comparison with dynamic susceptibility contrast-enhanced MRI[J].Stroke,2012,43(4):1 018-1 024.
[13] SILACHEV D N,GULYAEV M V,ZOROVA L D,et al.Magnetic resonance spectroscopy of the ischemic brain under lithium treatment.Link to mitochondrial disorders under stroke[J].Chem Biol Interact,2015,237:175-182.
[13] YAN G,DAI Z,XUAN Y,et al.Early metabolic changes following ischemia onset in rats:an in vivo diffusion-weighted imaging and 1H-magnetic resonance spectroscopy study at 7.0 T[J].Mol Med Rep,2015,11(6):4 109-4 114.
[14] YU S,LIEBESKIND D S,DUA S,et al.Postischemic hyperperfusion on arterial spin labeled perfusion MRI is linked to hemorrhagic transformation in stroke[J].J Cereb Blood Flow Metab,2015,35 (4):630-637.
[15] SHINOHARA Y,KATO A,KUYA K,et al.Perfusion MR Imaging Using a3D Pulsed Continuous Arterial Spin-Labeling Method for Acute Cerebral Infarction Classified as Branch Atheromatous Disease Involving the Lenticulostriate Artery Territory[J].AJNR Am J Neuroradiol,2017,38(8):1 550-1 554.
[16] JIMÉNEZ-XARRIÉ E,DAVILA M,GIL-PEROTÍN S,et al.In vivo and ex vivo magnetic resonance spectroscopy of the infarct and the subventricular zone in experimental stroke[J].J Cereb Blood Flow Metab,2015,35(5):828-834.
[17] IGARASHI H,SUZUKI Y,HUBER V J,et al.N-acetylaspartate decrease in acute stage of ischemic stroke:a perspective from experimental and clinical studies[J].Magn Reson Med Sci,2015,14(1):13-24.
[18] LEHMAN L L,RIVKIN M J.Perinatal arterial ische-mic stroke:presentation,risk factors,evaluation,and outcome[J].Pediatr Neurol,2014,51(6):760-768.
[19] WETTERLING F,GALLAGHER L,MULLIN J,et al.Sodium-23 magnetic resonance imaging has potential for improving penumbra detection but not for estimating stroke onset time[J].J Cereb Blood Flow Metab,2015,35(1):103-110.
[20] ALLIBERT R,BILLON GRAND C,VUILLIER F,et al.Advantages of susceptibility-weighted magnetic resonance sequences in the visualization of intravascular thrombi in acute ischemic stroke[J].Int J Stroke,2014,9(8):980-984.
[21] LEIGH R,KRAKAUER J W.MRI-guided selection of patients for treatment of acute ischemic stroke[J].Curr Opin Neurol,2014,27(4):425-433.
[22] DANI K A,WARACH S.Metabolic imaging of ischemic stroke:the present and future[J].AJNR Am J Neuroradiol,2014,35(6 Suppl):S37-S43.
[23] BARBER P A.Magnetic resonance imaging of ischemia viability thresholds andthe neurovascular unit[J].Sensors (Basel),2013,13(6):6 981-7 003.
[24] CRACIUNAS S C,BROOKS W M,NUDO R J,et al.Motor and premotor cortices in subcortical stroke:protonmagnetic resonance spectroscopy measures and arm motor impairment[J].Neurorehabil Neural Repair,2013,27(5):411-420.
[25] SINGHAL A B,RATAI E,BENNER T,et al.Magnetic resonance spectroscopy study of oxygen therapyin ischemic stroke[J].Stroke,2007,38(10):2 851-2 854.
[26] DAVIS D P,ROBERTSON T,IMBESI S G.Diffusion-weighted magnetic resonanceimaging versus computed tomography in the diagnosis of acute ischemic stroke[J].J Emerg Med,2006,31(3):269-277.
[27] KARASZEWSKI B,WARDLAW J M,MARSHALL I,et al.Measurement of brain temperature with magneticresonance spectroscopy in acute ischemic stroke[J].Ann Neurol,2006,60(4):438-446.
[28] WEBER R,RAMOS-CABRER P,HOEHN M.Present status of magnetic resonance imaging and spectroscopy in animal stroke models[J].J Cereb Blood Flow Metab,2006,26(5):591-604.
[29] FISHER M,PRICHARD J W,WARACH S.New magnetic resonance techniques for acute ischemic stroke[J].JAMA,1995,274(11):908-911.
[30] RYU J A,BANG O Y,LEE G H.D-dimer levels and cerebral infarction in criticallyill cancer patients[J].BMC Cancer,2017,17(1):591.doi:10.1186/s12885-017-3588-7.
[31] ONO H,NISHIJIMA Y,OHTA S,et al.Hydrogen Gas Inhalation Treatment in Acute Cerebral Infarction:A Randomized Controlled Clinical Study on Safety and Neuroprotection[J].J Stroke Cerebrovasc Dis,2017,26(11):2 587-2 594.doi:10.1016/j.jstrokecerebrovas-dis.2017.06.012.
[32] DESILLES J P,SYVANNARATH V,OLLIVIER V,et al.Exacerbation of Thromboinflammation by Hyperglycemia Precipitates Cerebral Infarct Growth and Hemorrhagic Transformation[J].Stroke,2017,48(7):1 932-1 940.doi:10.1161/STROKEAHA.117.017080.
[33] KATO A,SHINOHARA Y,KUYA K,et al.Proximal Bright Vessel Sign on Arterial Spin Labeling Magnetic Resonance Imaging in Acute Cardioembolic Cerebral Infarction[J].J Stroke Cerebrovasc Dis,2017,26(7):1 457-1 461.doi:10.1016/j.jstrokecerebrovasdis.2017.03.015.
[34] MORITA Y,KATO T,OKANO M,et al.Incidence and Predictors of Catheterization-Related Cerebral Infarction on Diffusion-Weighted Magnetic Resonance Imaging[J].Biomed Res Int,2016:6 052 125.doi:10.1155/2016/6052125.
[35] XU R,YIN X,XU W,et al.Assessment of carotid plaqueneovascularization by contrast-enhanced ultrasound and high sensitivityC-reactive protein test in patients with acute cerebral infarction:a comparativestudy[J].Neurol Sci,2016,37(7):1 107-1 112.doi:10.1007/s10072-016-2557-2.
[36] LIN Z,GUO Z,QIU L,et al.The applied research of MRI withASSET-EPI-FLAIR combined with 3D TOF MRA sequences in the assessment of patientswith acute cerebral infarction[J].Acta Radiol,2016,57(12):1 515-1 523.doi:10.1177/0284185116628338.
[37] KORBAKIS G,PRABHAKARAN S,JOHN S,et al.MRIDetection of Cerebral Infarction in Subarachnoid Hemorrhage[J].Neurocrit Care,2016,24(3):428-435.doi:10.1007/s12028-015-0212-z.
[38] CHUNG J W,PARK S H,KIM N,et al.Trial of ORG 10172 in Acute Stroke Treatment(TOAST) classifi-cation and vascular territory of ischemic stroke lesionsdiagnosed by diffusion-weighted imaging[J].J Am Heart Assoc,2014,3(4).pii:e001119.doi:10.1161/JAHA.114.001119.
(收稿2018-09-11 修回2018-10-12)
本文责编:王喜梅
本文引用信息:卢明聪,符大勇,孟云,周建国,马先军,刘晓丽.表观弥散系数及脑血流量值在急性大面积缺血性脑卒中出血转化预测中的应用价值[J].中国实用神经疾病杂志,2018,21(20):2205-2211.DOI:10.12083/SYSJ.2018.20.477
Reference information:LU Mingcong,FU Dayong,MENG Yun,ZHOU Jianguo,MA Xianjun,LIU XiaoLi.Value of apparent diffusion coefficient and cerebral blood flowin predicting hemorrhagic transformation in acute massive ischemic stroke[J].Chinese Journal of Practical Nervous Diseases,2018,21(20):2205-2211.DOI:10.12083/SYSJ.2018.20.477